|
This is pretty classic FRCR case. It is a good test of observational skills. If you are alert, several findings will lead to the diagnosis. However, this is the kind of case where a directed search for other findings, based on identification of one or two inital findings is most fruitful.
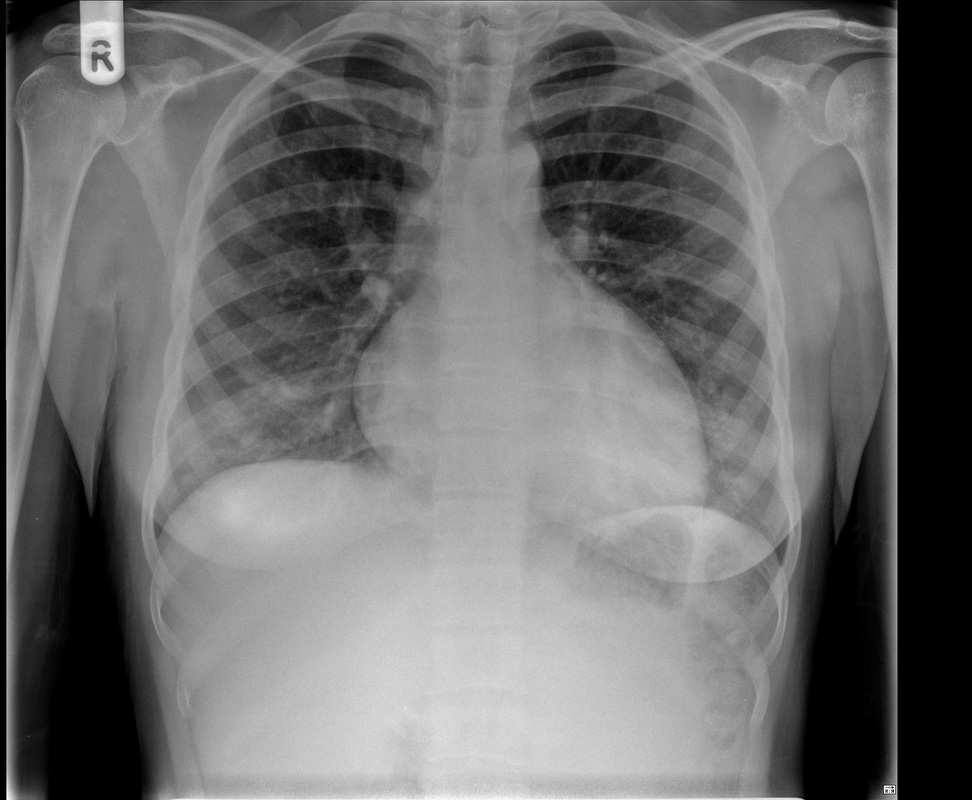
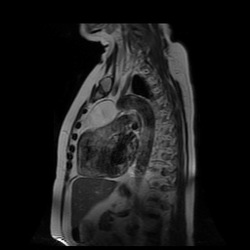
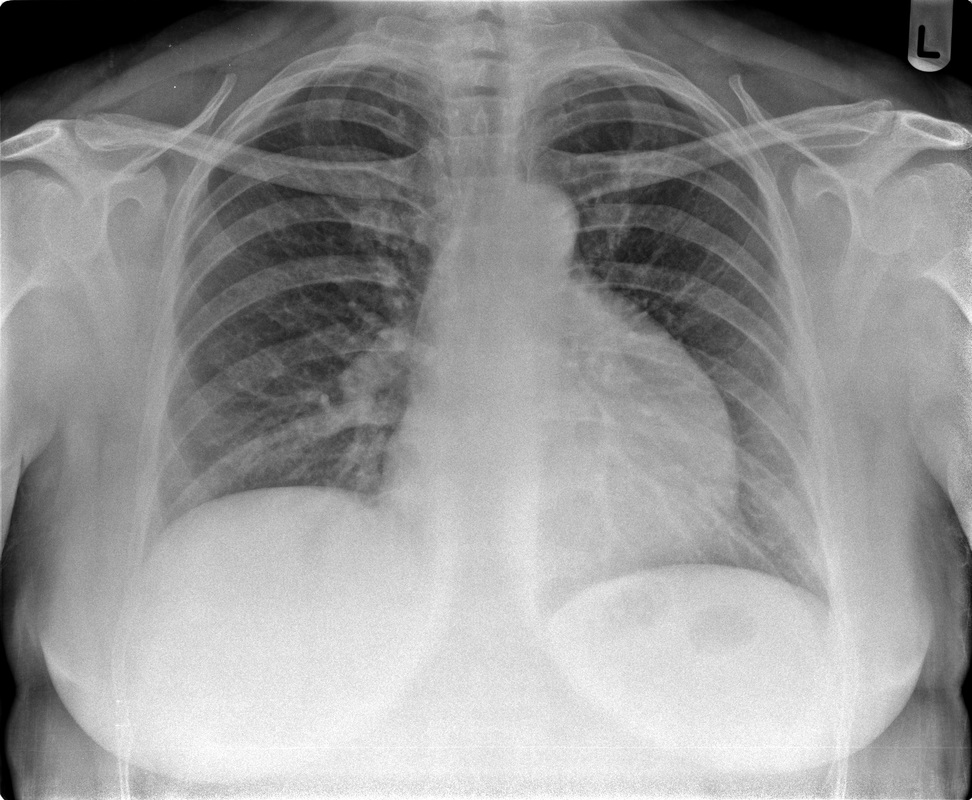
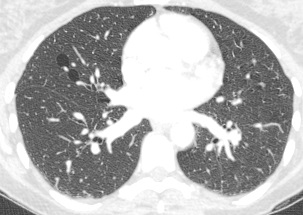
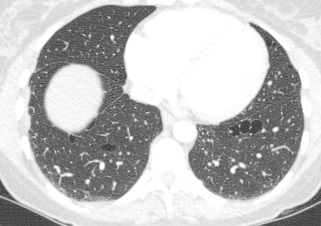
Perhaps the most obvious finding is that there is mild cardiomegaly, Careful review of the lung parenchyma suggests pulmonary vasculature may also be slightly prominent. These are unusual finding for a patient aged 20. This might suggest cardiac or renal impairment.Consideration of renal impairment should prompt evaluation of osseous structures, to determine whether there is mild osseous sclerosis that might indicate renal osteodystrophy. There is questionable mild sclerosis present, however, more specifically evaluation of the lower thoracic/upper lumbar spine demonstrates that there is multilevel mild central endplate depression. This finding, often referred to as H-shaped vertebral bodies is relatively characteristic findings of sickle cell disease due to microvascular end plate infarction. In turn identification of this finding should on search for further findings that would corroborate the diagnosis of sickle cell disease. One might consider evaluating the gallbladder for gallstones. In this patient surgical clips are present, consistent with a cholecystectomy, again unusual in a patient of this age, unless the patient has sickle cell disease, confirming our diagnosis. Further considerations may include evaluation of the left upper quadrant to identify a very small or densely calcified spleen, not clearly visible in this case. Further review of the osseous structures should extend to evaluate whether there are sclerotic changes of the humeral head which may reflect early avascular necrosis, again not present in this case. The cardiac features may relate to a variety of causes, including left heart failure due to myocarditis/cardiomyopathy, including ischaemic changes or relate to right cardiac impairment. Right cardiac impairment may be secondary to recurrent pulmonary parenchymal infection/scarring, acute or chronic thromboembolic disease or microvascular peripheral pulmonary arterial disease. There is an interesting case because it highlights that although a repeatable systematic approach to the evaluation of chest radiographs, other radiographs or cross-sectional imaging has its value, it should be as an adjunct to directed evaluation of focal areas based on the principle that when a finding is present, consider and look for what may have caused that finding and consider also what that finding may lead onto. Considering films in this way greatly increases your diagnostic interpretation quality. The original chest x-ray is nearly normal. There is prominence of the left cardiac border which appears wider and more convex than normal. Initial considerations for this may include the possibility of a pectus excavatum or loss of the normal thoracic kyphosis ("straight back syndrome"). However, in these instances there is usually a vertical orientation of the anterior ribs and in addition the right cardiac silhouette is displaced behind the spine. Similarly rarer instances of complete or partial pericardial absence can also cause prominence of the left cardiac border but again this is also usually associated with loss of the right cardiac border behind the spine.
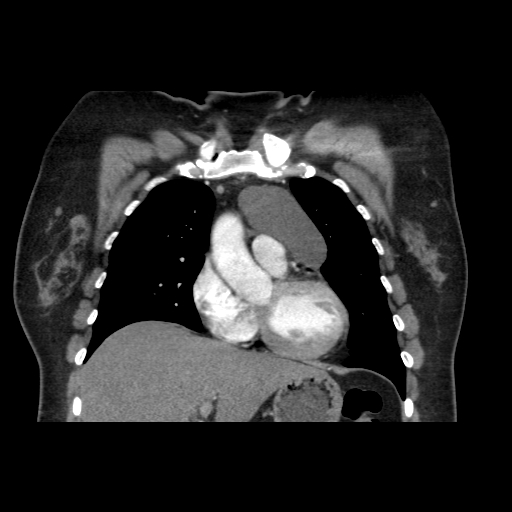
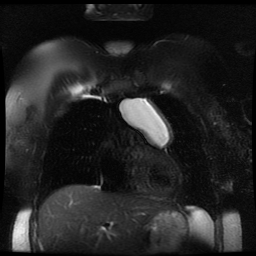
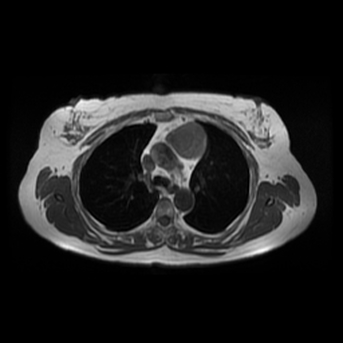
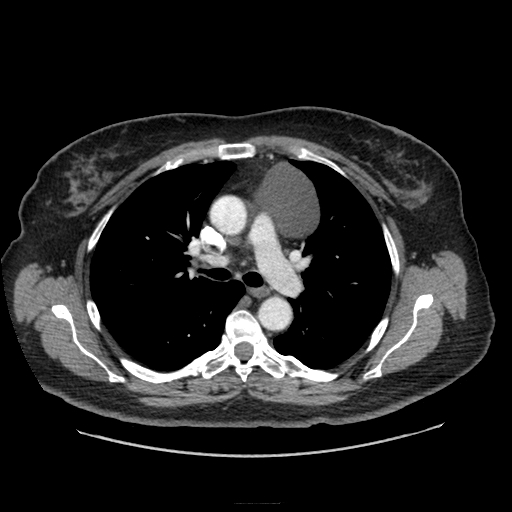
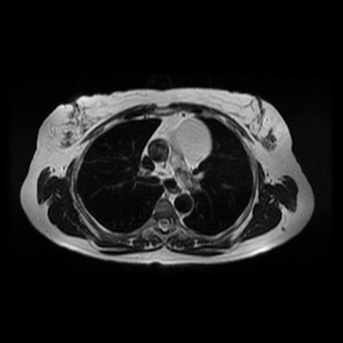
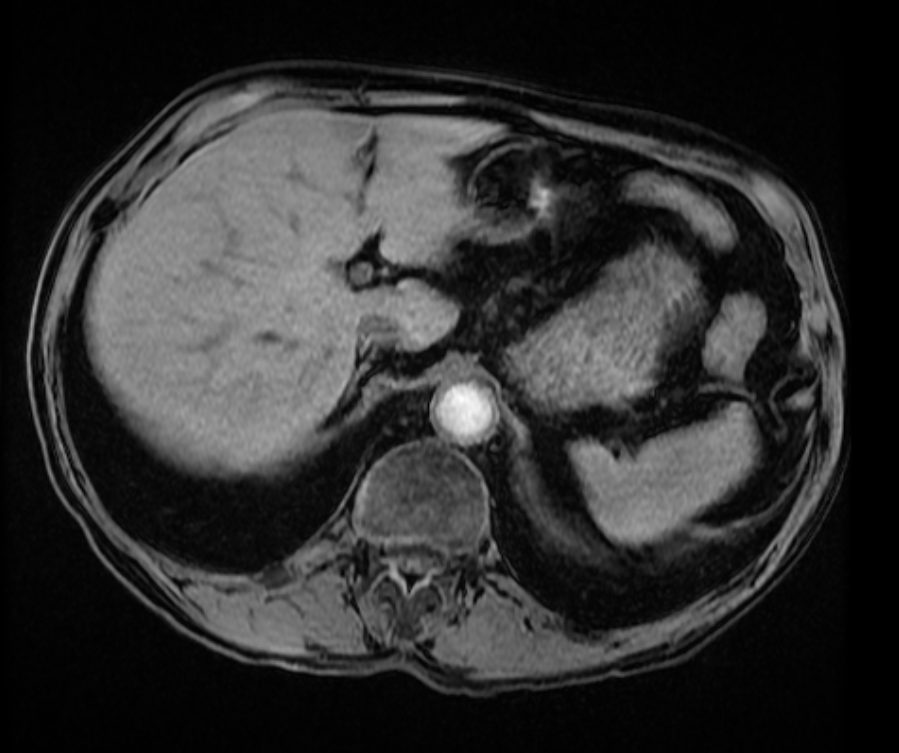
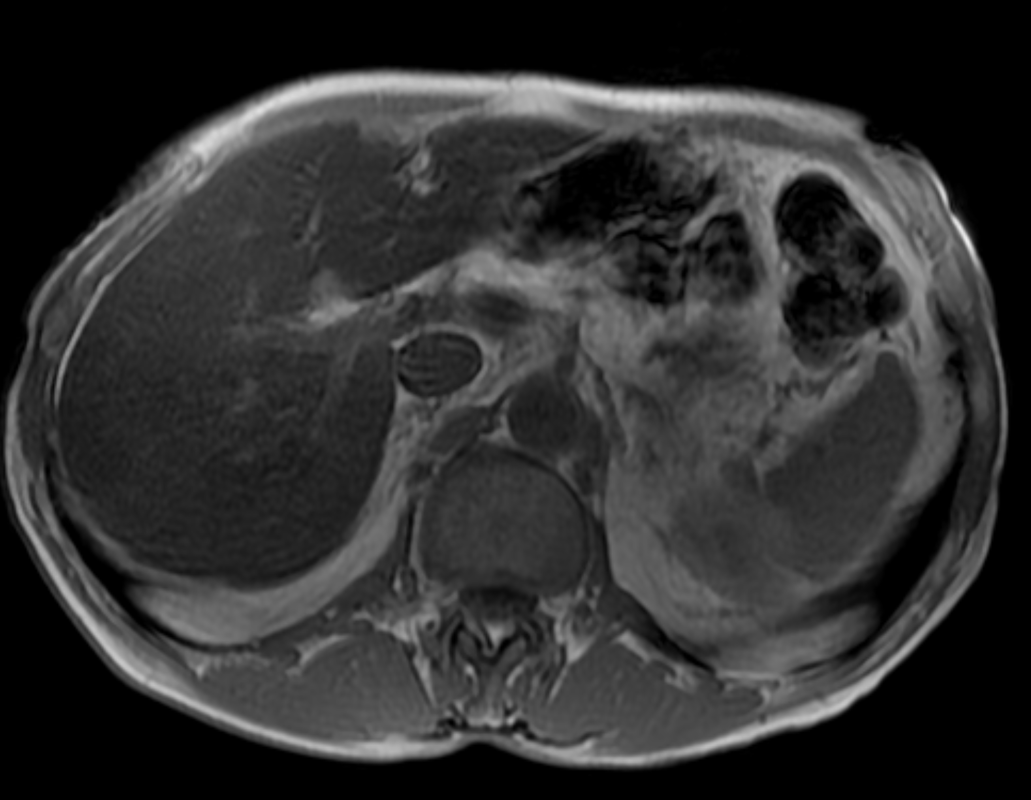
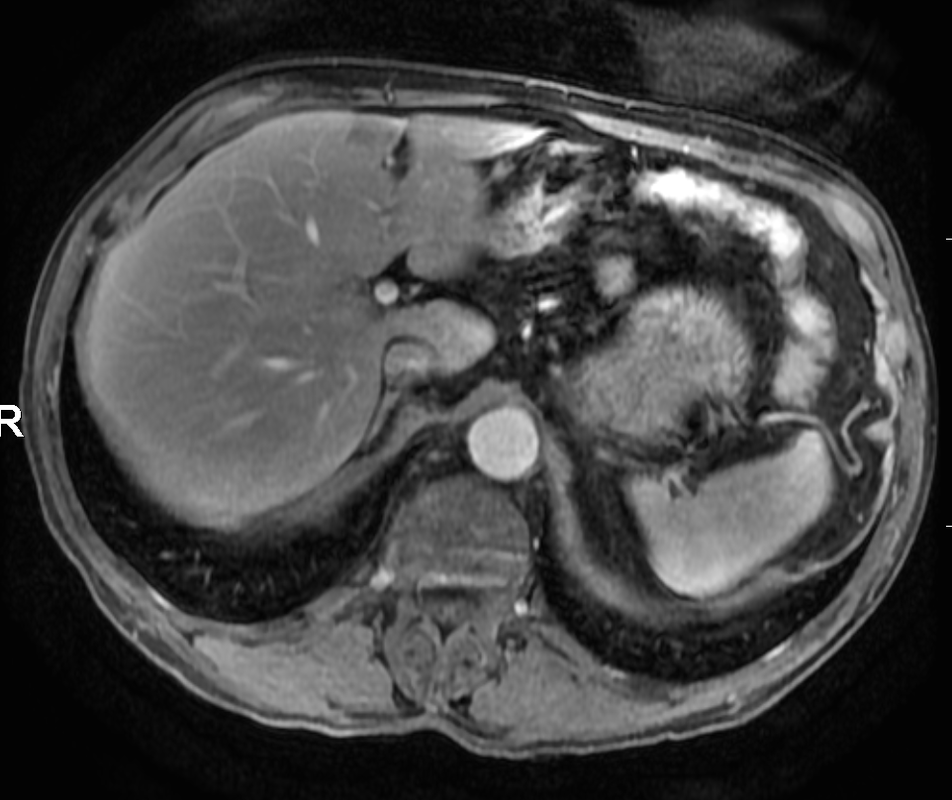
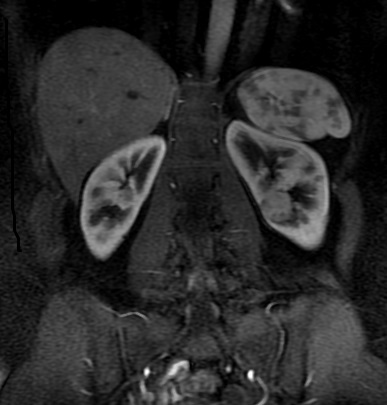
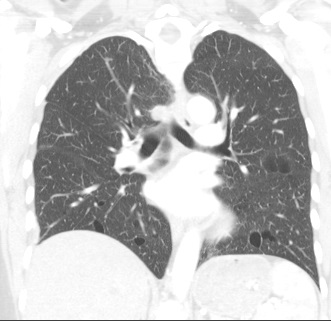
Other considerations might include the possibility of a cardiac, pericardial or mediastinal mass. This is an unusual location for a cardiac abnormality. A left ventricular aneurysm is usually more inferior. Occasional but very rare corrected transposition of the great vessels can result in prominence of the left cardiac/mediastinal border, however, is associated with a narrow superior mediastinum due to superimposition of the aorta and pulmonary artery (so called "egg on a string" ). Pericardial abnormalities include the possibility of masses and more commonly a pericardial cyst, for which this appearance is a possibility. Mediastinal abnormalities include most anterior mediastinal masses in particular in this location extending inferiorly over the cardiac right or left sided silhouette the possibility of a thymic mass. CT imaging demonstrates that the epicentre of the lesion is higher within the anterior mediastinum and that the lesion lesion is uniformly of low attenuation. There is a comparable cystic appearance at MRI with low T1 and high T2 signal. No peripheral soft tissue is appreciated. Post gadolinium acquisitions (not demonstrated) demonstrated no enhancement within the lesion or at its margins. The location of the lesion favours a mediastinal abnormality rather than a pericardial lesion. The diffuse cystic nature of the lesion is likely due to a thymic cyst. Thymic cysts may be cervical, mediastinal or a combination. Typically these demonstrate entirely cystic appearances, although the typical low T1/ high T2 signal may be affected by some variation in this signal according to variable turbidity and the often gelatinous nature of the contents of the cyst. Usually these are unilocular but multilocular lesions can occur too. Thymic cysts are benign lesions which may be congenital or acquired, the latter usually occurring as the result of treatment of mediastinal lymphoproliferative disorders. They are also described as a postoperative complication although typically fluid collection in the anterior mediastinum are not within the thymus itself. Thymic cysts are thought to reflect congenital cystic transformations of ductal epithelial formations of residual branchial pouch elements. Histological sampling may demonstrate walls with squamous, columnar or cuboidal epithelium with thymic resisual tissue. The walls demonstrate areas of inflammation, haemorrhage, fibrosis or granulation tissue. Typical Hassall's corpuscles in the cyst wall may be identified, more commonly in congenital lesions. Thymic cysts require no specific treatment unless there are enlarging or causing mass effect. Reports of rapidly enlarging thymic cysts are usually due to haemorrhage within the lesion which would necessitate surgery for diagnosis and treatment. MRI may be of assistance in differentiating the rarer instance of a cystic thymoma or near complete cystic germ cell tumour. The absence of a peripheral rim of soft tissue or septation strongly and at the case against those diagnoses in this patient. Case to Ponder 37 Answer: Focal Fatty Infiltration on a background of Diffuse Haemosiderosis21/11/2015 T1 fat suppressed images before and after intravenous gadolinium demonstrate an area of relatively low precontrast T1 signal adjacent the falciform ligament. This area remains relatively hypointense compared to the background hepatic parenchyma on postcontrast imaging. This is a common site of differential focal fatty infiltration or sparing, likely due to differential perfusion in this region, often by branches of the left gastric artery or venous drainage into systemic veins.
The T1 in phase images demonstrate that there is high signal in this region and the paired out of phase images demonstrate that this region significantly loses signal, consistent with focal fatty infiltration. Is this all? The background hepatic parenchymal signal remainder of the liver is higher on the out of phase imaging than the in-phase imaging. This is unusual. Typically in a normal liver signal remains constant. In diffuse fatty infiltration, signal loss would be expected in areas of fatty infiltration. In this instance, however, the inverse is demonstrated for the bulk of the hepatic parenchyma. Why does this occur? This is due to hepatic haemosiderosis in the remainder of the liver. In phase imaging has a longer TE(time to echo) than out of phase imaging. As a result there is more time for Iron atoms to result in local dephasing on in phase imaging. In turn this manifests as loss of signal compared to out of phase imaging. So think of hepatic haemosiderosis as the inverse of hepatic steatosis with regards to liver in and out of phase imaging. In this case they can be to differentiated as they are occuring in distinct areas. If they are occurring in the same area of hepatic parenchyma combined defects can be difficult to differentiate and will depend on the extent of either present. What is the differential signal adjacent to the falciform ligament due to? Careful!
|
From Grayscale
Latest news about Grayscale Courses, Cases to Ponder and other info Categories
All
Archives
October 2018
|

|
|
Grayscale Courses est. 2015

 RSS Feed
RSS Feed
