|
Radiology Level: FRCR, FRCS, MRCP, ABR, EDiR, Radiology Senior +++
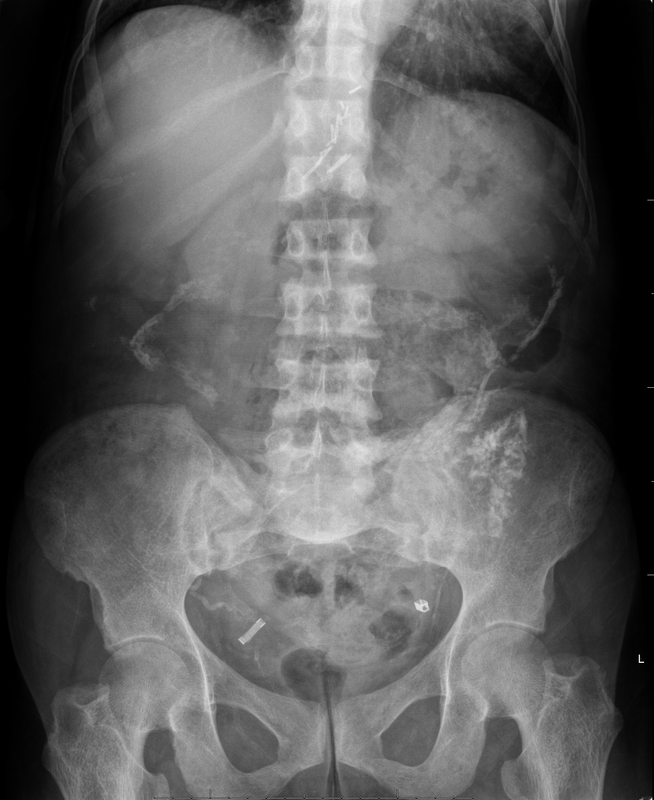
An interesting case to analyse. The abdominal radiograph demonstrates extensive areas of intrabdominal calcification. These do not conform to any intrabdominal organ, nor the bowel to suggest bowel contents. Therefore, these findings are likely peritoneal in nature. There are not many causes of peritoneal calcification. Intuitively, tuberculosis and peritoneal mesothelioma may come to mind. However, in the former calcification is unusual and limited and localised when present. Calcification is even rarer within peritoneal mesothelioma even if pleural plaques are calcified in the same patient. So it is important to consider other conditions. Pseudomyxoma peritonei can be associated with small areas of focal punctate or linear calcification. In these cases the bowel would be expected to be centrally displaced by the gelatinous soft tissue filling the peritoneum. This is not present in this case. Two conditions should be considered with extensive calcifications of this type. These are psammomatous calcifications in ovarian malignancy and the calcifications of sclerosing peritonitis. These two types of calcifications can be differentiated by their morphology. Psammoma bodies are usually fluffy calcifications that appear almost to be forming ossified bodies. In distinction the calcfications of sclerosing peritonitis are usually linear or sheet like. Therefore, in this case the calcifications are typical of sclerosing (or encapsulated) peritonitis. This is a calcification pattern that develops in patients on peritoneal dialysis. The appearances are often referred to as a "cocoon abdomen". The presence of the calcifications and peritoneal loculations impairs the ability to perform peritoneal dialysis effectively by reducing the peritoneal surface accessible to the dialysate fluid. When identifying features of one disease it is also important to look for further features that may corroborate the process. What about the bones, are they normal? The bone density is diffusely increased, the trabeculae are ill-defined, there is alternate sclerosis and lucency of the vertebral bodies ("rugger jersey spine"). These are features of renal osteodystrophy. The sacro-iliac joints are also ill-defined and widened. This is a feature of the hyperparathyroidism component of renal osteodystrophy resulting in subperiosteal bone resorption. Finally, vascular calcifications are present. These are less specific and seen not only in renal impairment but also in diabetes and elderly patients with atherosclerosis. The surgical clips in the pelvis are not related to a prior transplant as suggested by some, but rather due to sterilisation clips. Unrelated clips in the upper abdomen are due to unrelated surgery. Always look at all elements of a case. The bones, the soft tissues, the gas pattern, they may all assist in confirming a diagnosis and finding all its manifestations. Radiology Level: FRCR, FRCS, MRCP, ABR, EDiR, Radiology Senior +++
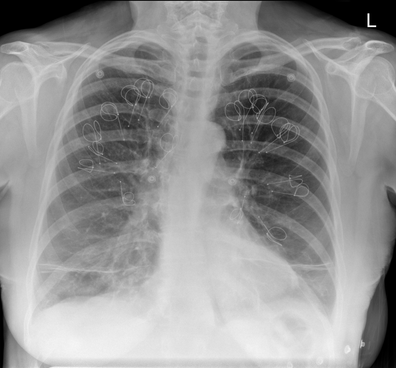
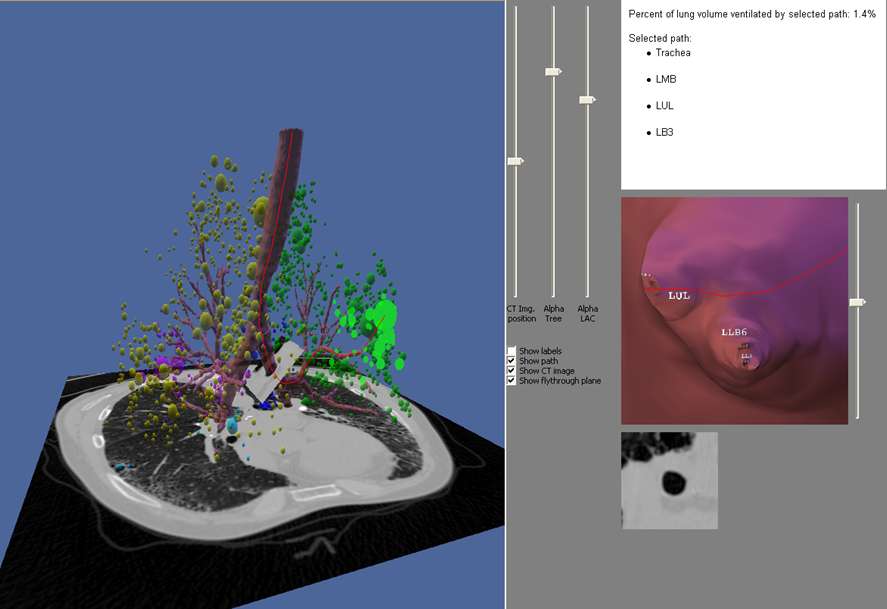
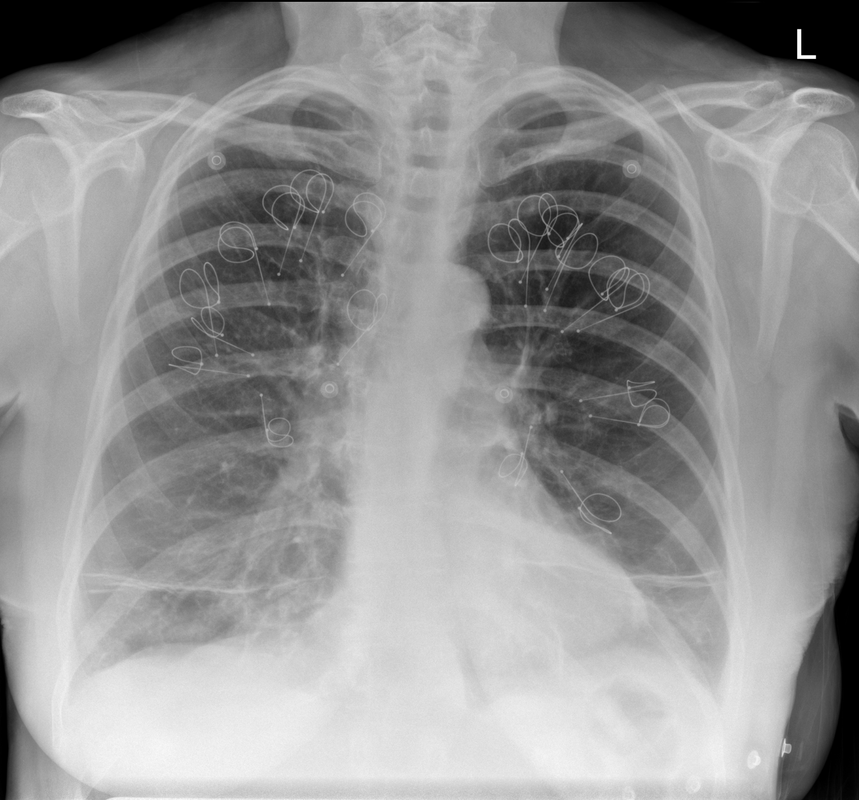
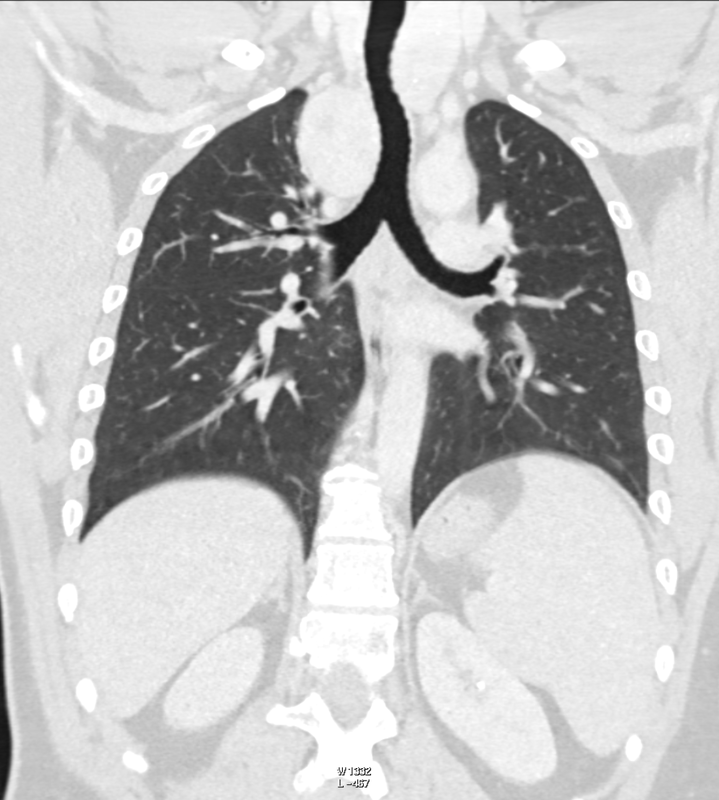
Ignore upper abdominal surgical clips. Radiology Level: FRCR, ABR, EDiR, MRCP, Radiology Senior +++ The appearances demonstrate multiple metallic loop structures in the upper lobes with extensive bibasilar atelectasis. These findings are due to endobronchial bronchoscopic insertion of nitinol endobronchial coils for the minimally invasive treatment of emphysema. The principal of usage of these colis is that by reducing air flow to emphysematous diseased segments of lung, that there will be improved air fluid to more functional lung parenchyma and overall this will result in improved gas exchange and hence lung function. The procedure is intended to improve lung function patients with upper or lower lobe heterogeneous emphysema. These endobronchial coils are inserted into the airways by bronchoscopy under sedation or general anaesthesia. A guide wire is inserted through the bronchoscope under fluoroscopic guidance. A catheter is passed over the guide wire and the straightened coil is introduced through the catheter with the catheter withdrawn as the coil held in place using grasper. When released the straightened coil reverts to to predetermined shape pulling the surrounding disease tissue and reducing lung volume. Usually up 5-15 coils are inserted per treated lobe (usually the upper lobes). Each lung is treated separately as there is a risk of pneumothorax. The coils remain permanently in location. This type of volume reduction surgery is an alternative to patients undergoing formal lung volume reduction surgery or endobronchial valve insertion. The validity of such usage has been supported by small trials that suggest principally improved symptomatology, rather than measurable improved pulmonary function, after insertion versus control patients. The usage has been supported in the UK by NICE guidance. My entirely anecdotal experience, supported by radiological imaging is that this is entirely bogus! Emphysema at CT is either focal, or diffuse and heterogeneous. If it is focal and compressing normal lung, lung volume reduction surgery makes sense and I have seen real improvement. Endobronchial valves should theoretically work but tend in my experience to become obstructed by lung becoming atelectatic adjacent to them. Both these procedures can be supported by lung CT that specifically guides by virtual bronchoscopy to the areas to resect, or the particular airways to obstruct or bypass by a valve (see image below). In contradistinction these valves may I am sure help the occasional patient who has diffuse emphysema in the upper lobes, by reducing ventilation and perfusion to these areas. But for most people with "heterogeneous emphysema" on CT have a bulla here and a bulla there throghout the lungs. Randomly inserting some coils is just as likely to reduce ventilation and perfusion to a good subsegment of lung as to an emphysematous portion.
It, therefore, comes to me as no surprise that a recent large study in JAMA casts doubt even on the symptom based improvement of these devices (6 minute walks, questionairres), suggesting much of this must be placebo related. Importantly it states that the treated groups unsurprsingly suffer considerably more pneuthoraces and infective exacerbations. Regardless these will be around for a while and need to be recognised. This patient suffered from recurrent haemoptysis, likely due the coils. Reference: Sciurba FC et al. JAMA. 2016 May 24-31;315(20):2178-89. doi: 10.1001/jama.2016.6261. Effect of Endobronchial Coils vs Usual Care on Exercise Tolerance in Patients With Severe Emphysema: The RENEW Randomized Clinical Trial. https://www.ncbi.nlm.nih.gov/pubmed/27179849 Radiology Level: FRCR, ABR, EDiR, FRCS, Radiology Senior +++
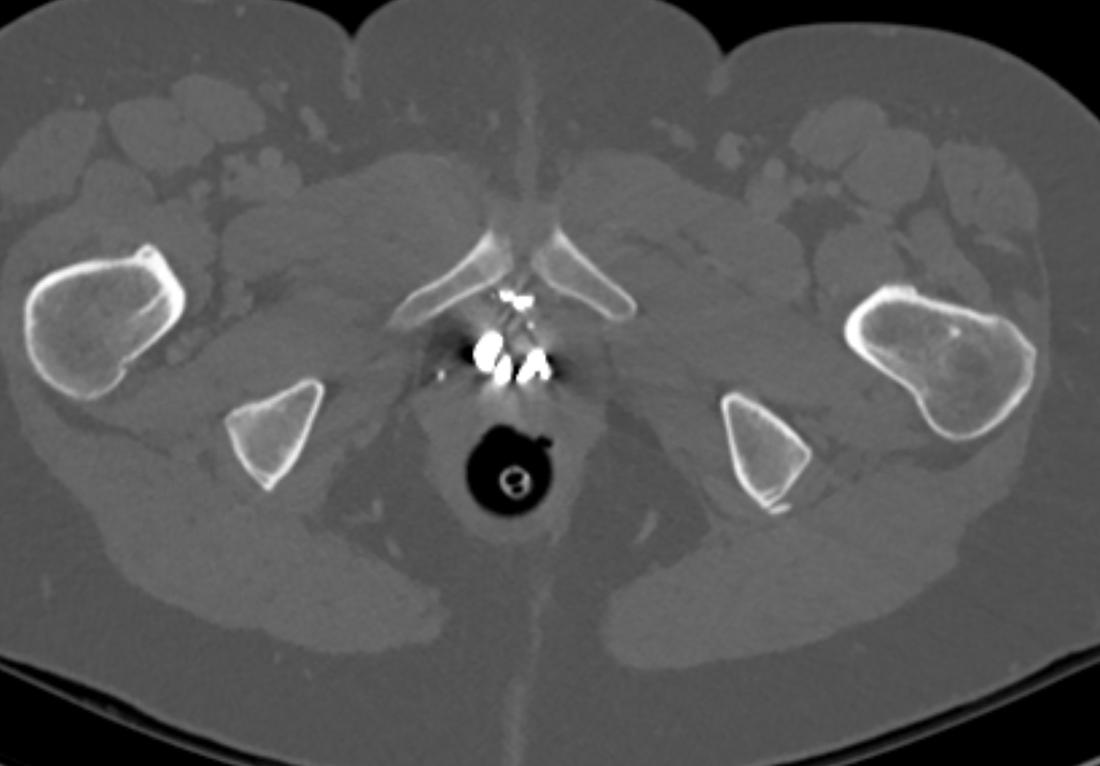
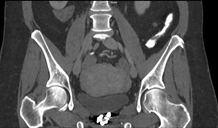
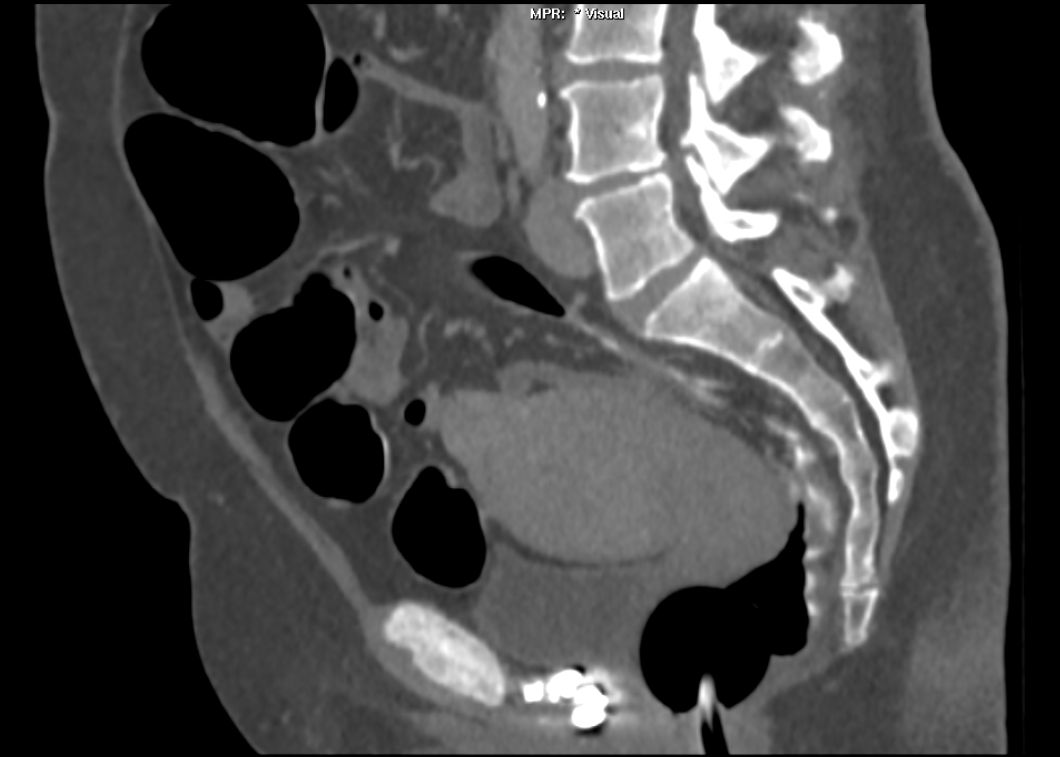
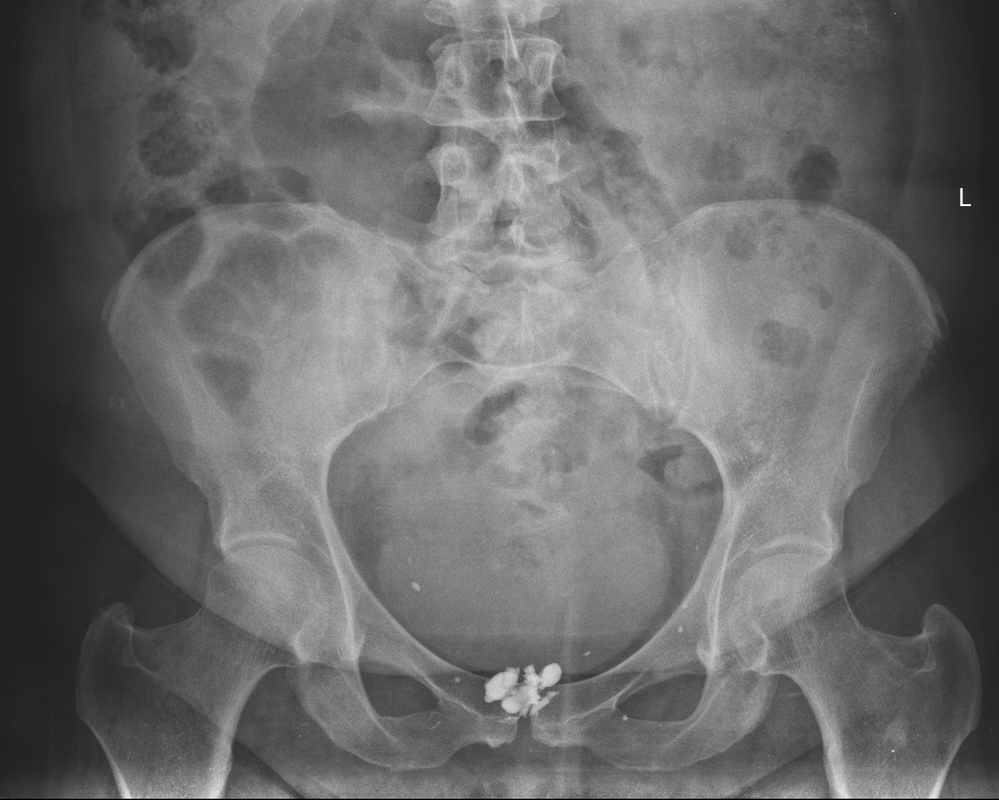
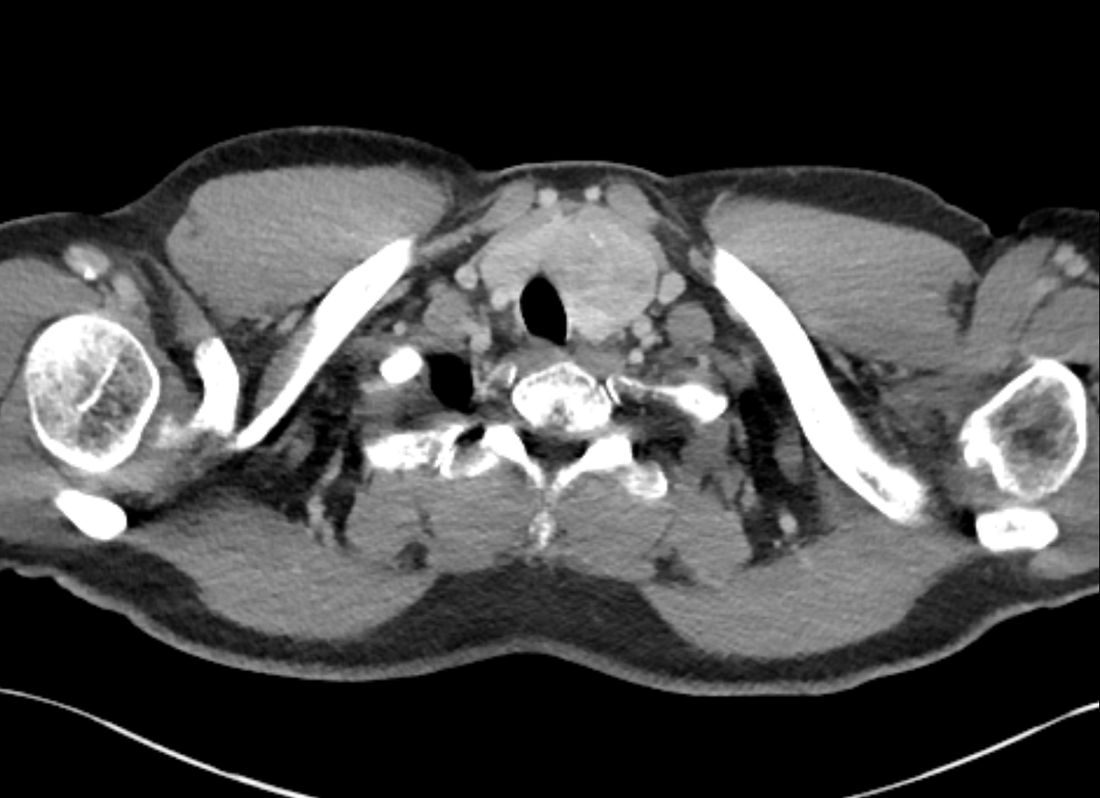
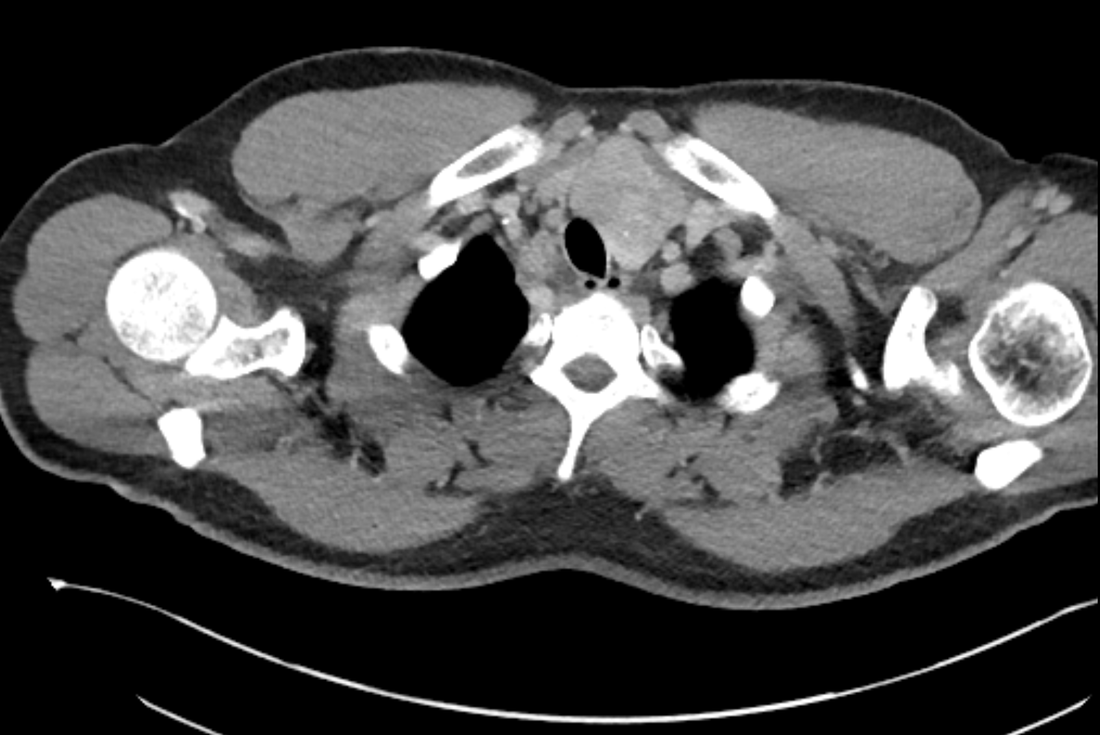
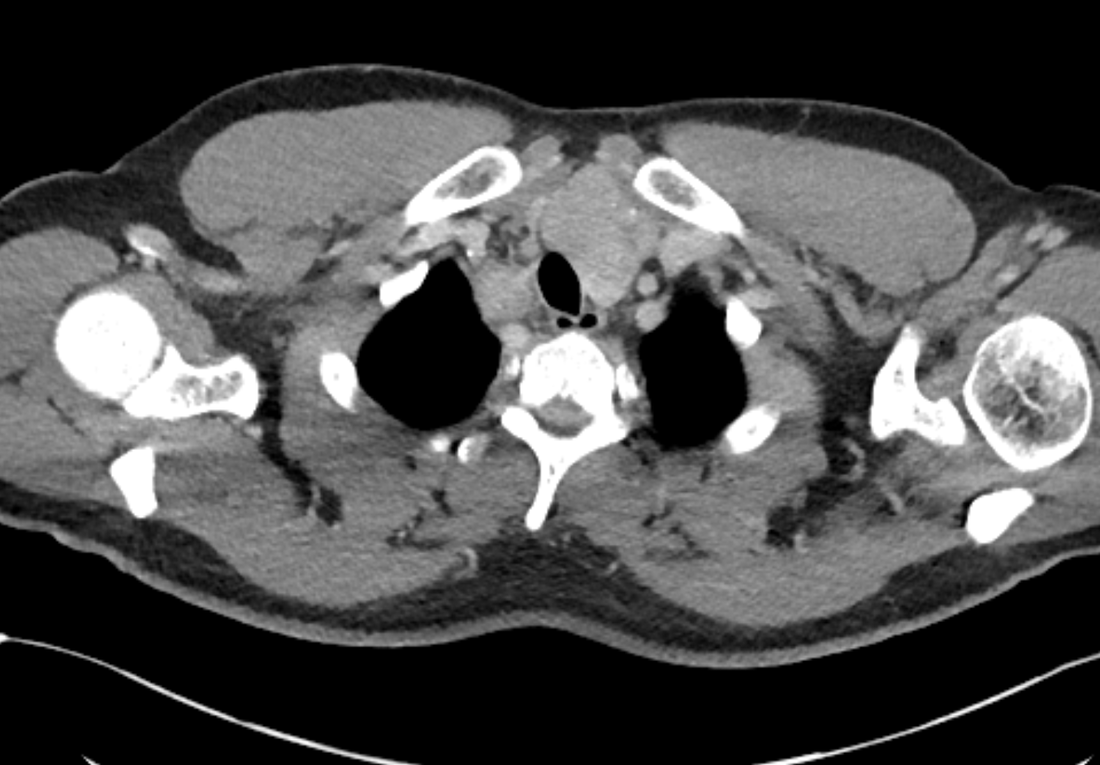
The images demonstrate high density clustered foci within the central lower pelvis. A few further foci noted in isolation along the pelvic sidewall. These appearances are too low central to reflect typical ureteral calculi and too inferior to reflect typical prostatic or seminal vesicle calcifications. The bladder outlines can be identified full of urine and the high density foci are noted to be below this, suggesting these are not bladder calculi. Indeed the density of these abnormalities far exceeds that of bone suggesting that these are metallic or other similar high density. The anatomic location is inferred from the abdominal radiograph but can be further delineated from the additional CT images provided. These demonstrate the presence of high density material below the bladder, surrounding the bladder neck. These appearances are due to periurethral injection of bulking agents in this case Duraspeheres. These agents are used to tackle stress urinary incontinence in female patients. In this instance the abnormality is due to a pyrolytic coated beads which stimulate collagen formation and improved urinary continence. The additional occasional sidewall high density focus is due to vascular extravasatiion which should be avoided. Bulking materials previously used included autologous fat. However, this has resulted in anecdotal fatal lipid pulmonary embolism and is no longer advised. Injections are targeted into the submucosal tissues of the urethra and bladder neck under anaesthesia. Radiology Level: FRCR, ABR, EDiR, MRCP, Radiology Senior +++
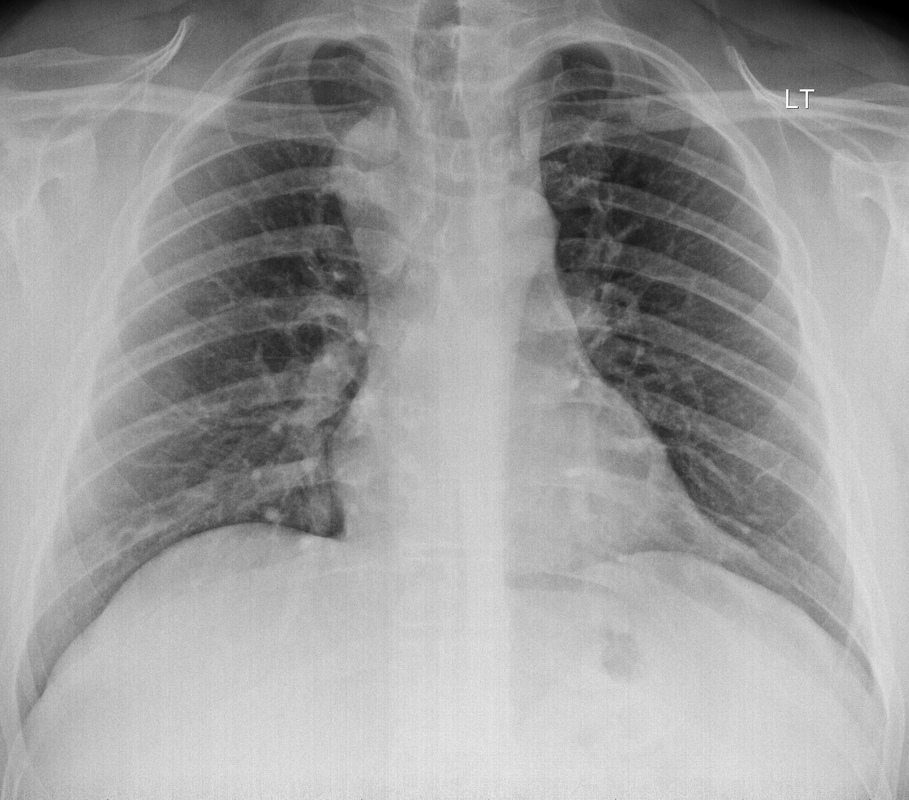
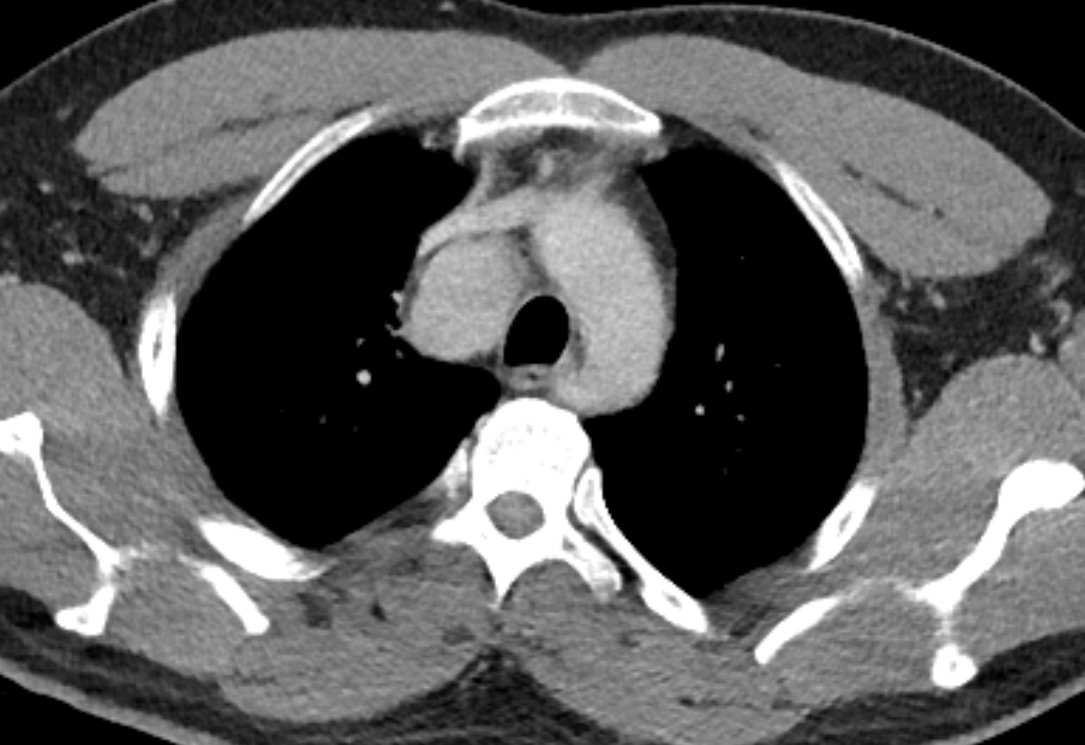
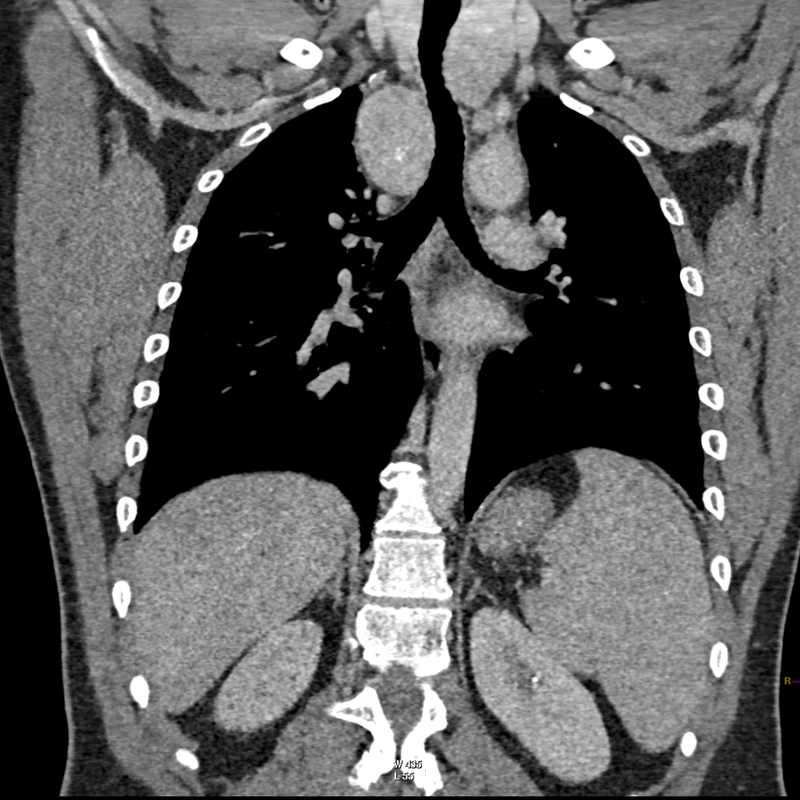
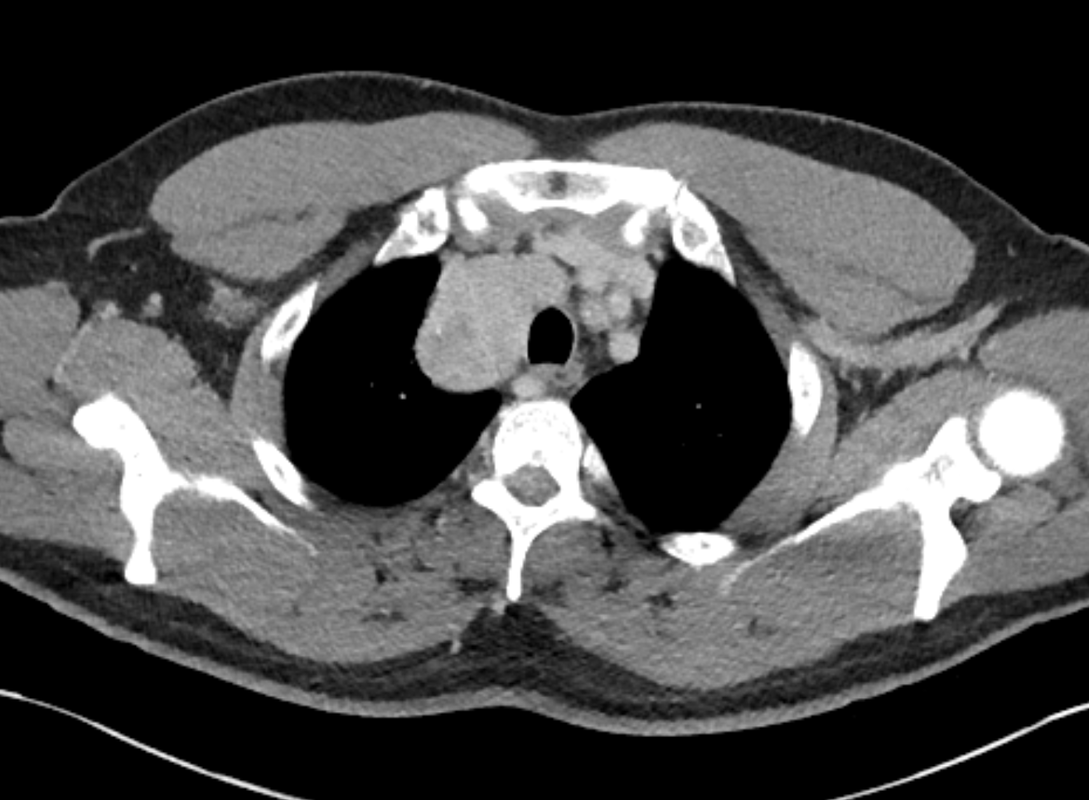
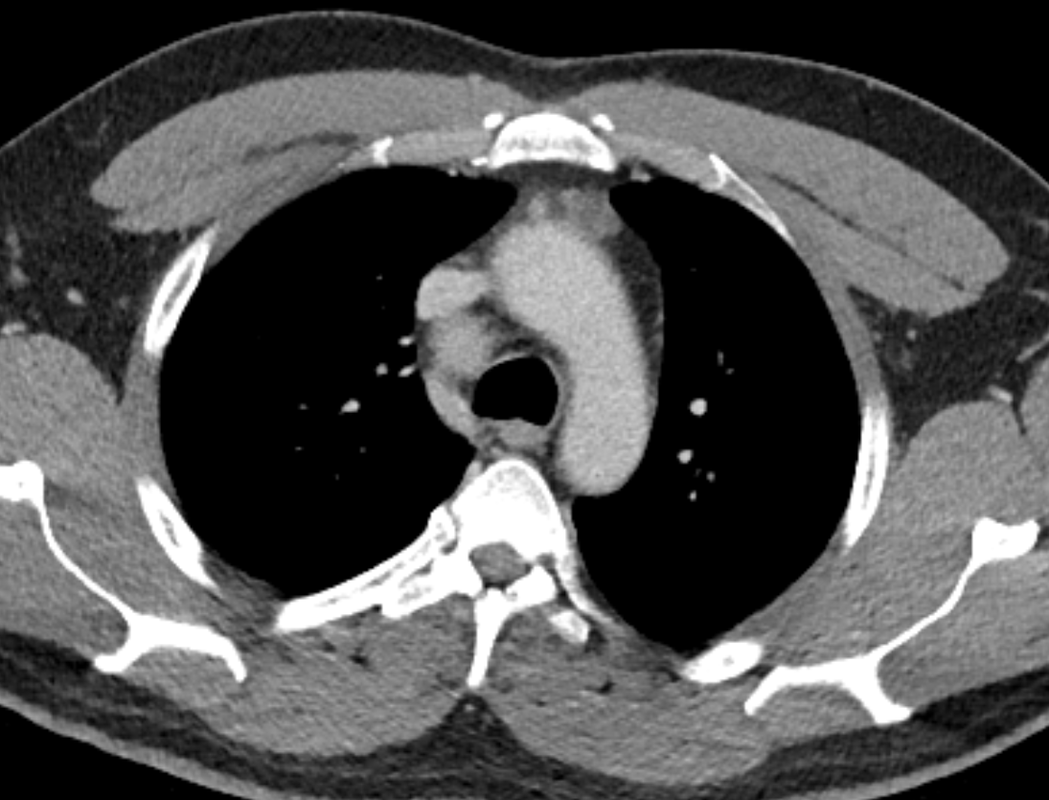
The initial chest x-ray demonstrates a right paramediastinal opacity confluent with and widening the right paratracheal stripe. Compare this case to case 75. In that case the right paramediastinal mass opacity did not efface and widen the right paratracheal stripe and so was not anatomically right paratracheal. The current case chest radiographic appearances suggest that the findings are due to a mass lesion adjacent to the right margin of the trachea. Is the rest of the chest x-ray normal? Well actually no it is not. Look at the more superior trachea. It is displaced to the right, suggesting a more superior cervical. The most common cause for this would be a thyroid goitre. Could this be causing both the left and right paratracheal abnormalities? Now let us review the CT imaging. We can see that there is enlargement of the left lobe of the thyroid and that accounts for the displacement of the trachea to the right. As suggested on the chest x-ray there is indeed a right paratracheal abnormality and this is confirmed on the coronal reconstructions explaining why the chest radiograph right paratracheal stripe is widened. In viewing the characteristics of the right paratracheal tissue it is clear that this has a very similar consistency to the left paratracheal thyroidal tissue, containing heterogenous low attenuation areas as well as a focal calcification. However, axial and coronal images demonstrate that there is no connection between the inferior margin of the right thyroid and the right paratracheal mass. What is the differential for these appearances? Well we can consider causes of anterior mediastinal masses. However, the appearances are too heterogenous for a diagnosis of lymphoma. Too lateral for a diagnosis of thymoma or a teratoma. In this location we should also consider a focal enlarged node, due to metastatic disease or perhaps due to granulomatous aetiology. However, no other nodes were present elsewhere. Other rarer lesions to consider would include ectopic parathyroid lesions or paraganglioma lesions, although both these are more typically hyperenhancing. So what does this leave is with? Well the lesion looks exactly like a thyroid goitre even though it is not connected to the right lobe. Well if it looks like a duck and quacks like a duck the saying is it usually is a duck! My thought was that this was ectopic thyroid with a goitre. The lesion processed to biopsy by endobronchial ultrasound for confirmation revealing typical colloid material suggestive of a thyroid goitre. The biopsy confirms a benign goitre in ectopic mediastinal thyroid tissue. Ectopic thyroid tissue is not uncommon, occurring during in the embryonal migration of thyroid tissue from the base of the foramen caecum to the typical pretracheal region. However inferior mediastinal ectopy is far more uncommon, likely less than 1% of ectopic cases, although the right paratracheal location has been reported. This should be differentiated from a multinodular thyroid gland with a small atretic band from the right lobe to the right paratracheal mass. Typical thyroid goitres extending from the neck descend in the right paratracheal or left paratracheal location. It is usually only the rarer isthmic lesions that extend anteriorly into the anterior mediastinum as the list books suggest. This case was primarily included as a companion training exercise for case 75 to evaluate the right paratracheal region on chest radiographs. Compare these chest radiographs, the corresponding coronal CTs, to improve your interpretation of the right paratracheal region. |
From Grayscale
Latest news about Grayscale Courses, Cases to Ponder and other info Categories
All
Archives
October 2018
|

|
|
Grayscale Courses est. 2015


 RSS Feed
RSS Feed
