|
Original 2 Day FRCR Viva Course:
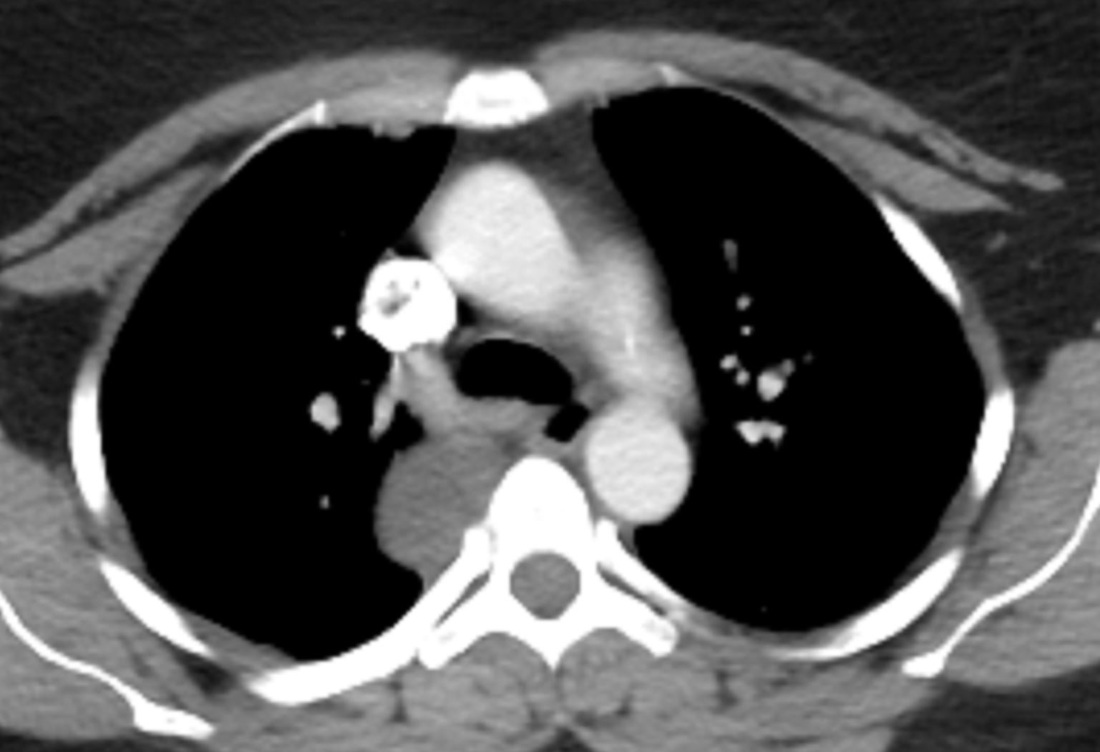
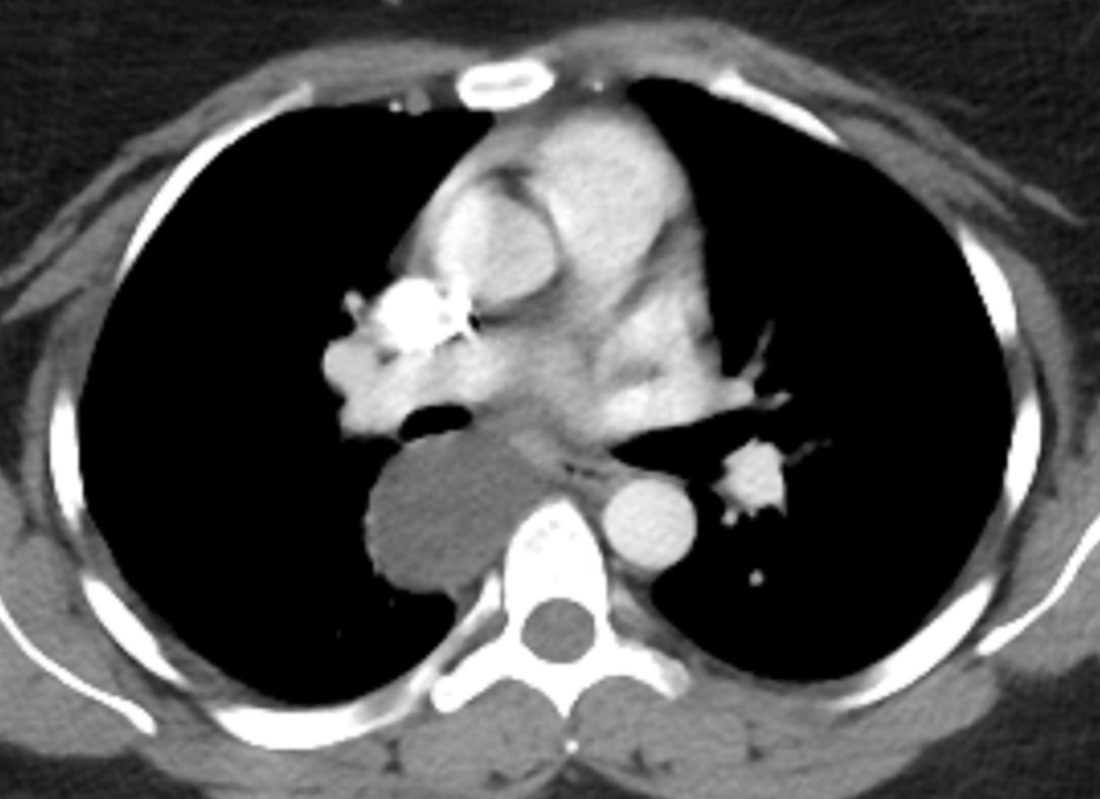
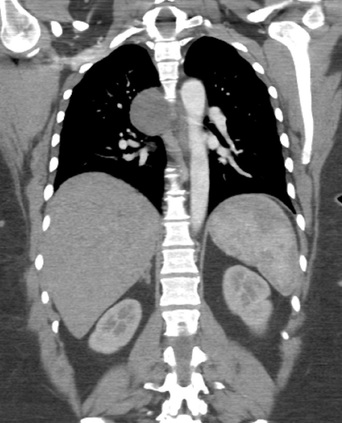
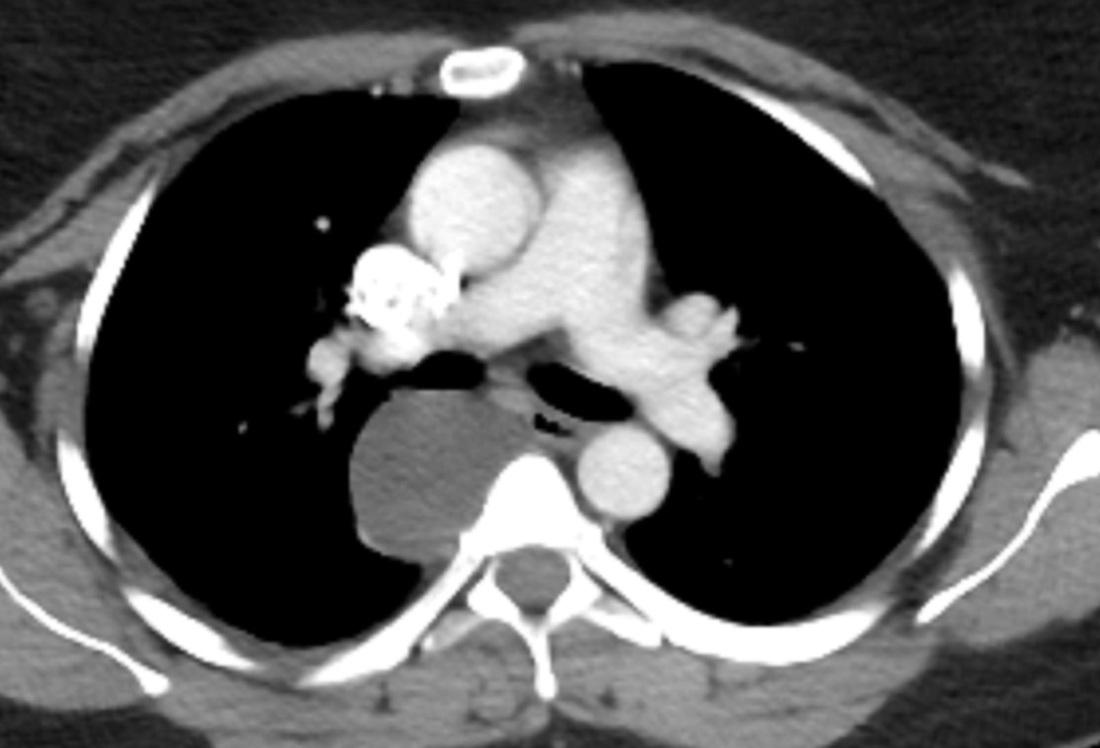
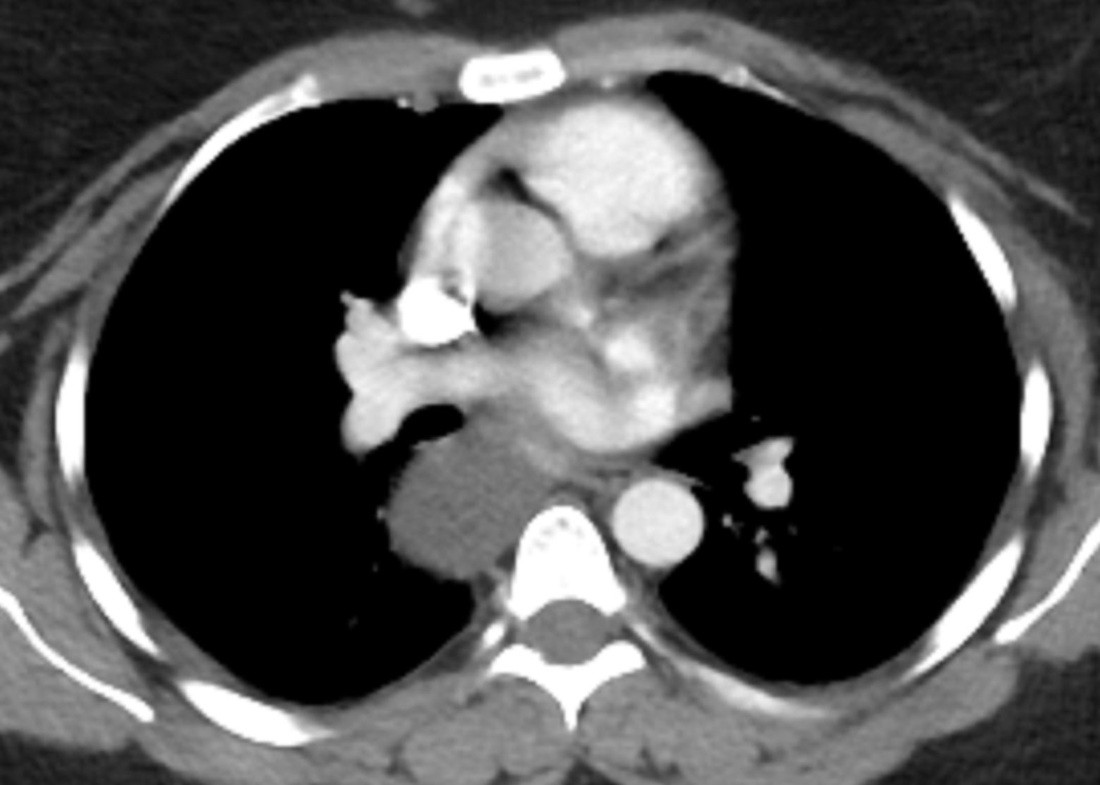
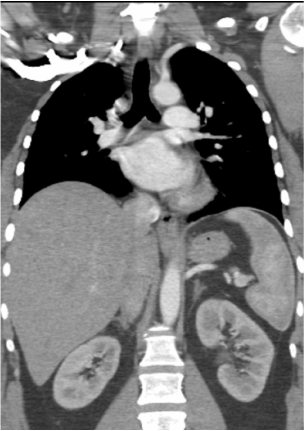
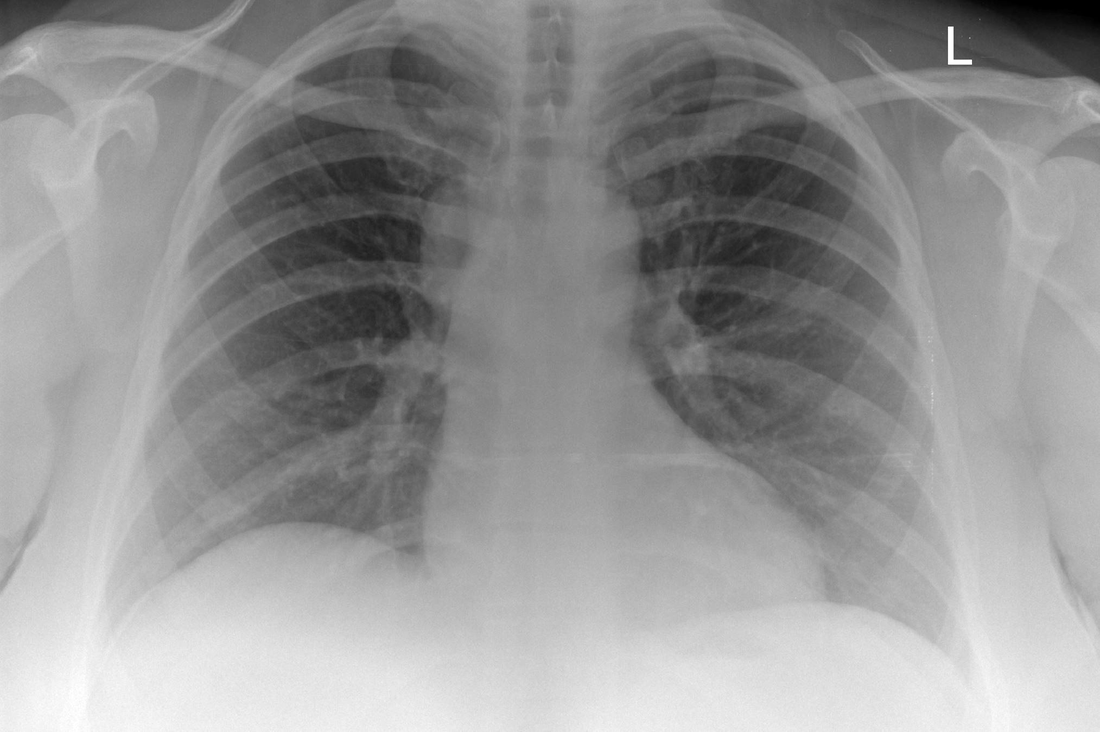
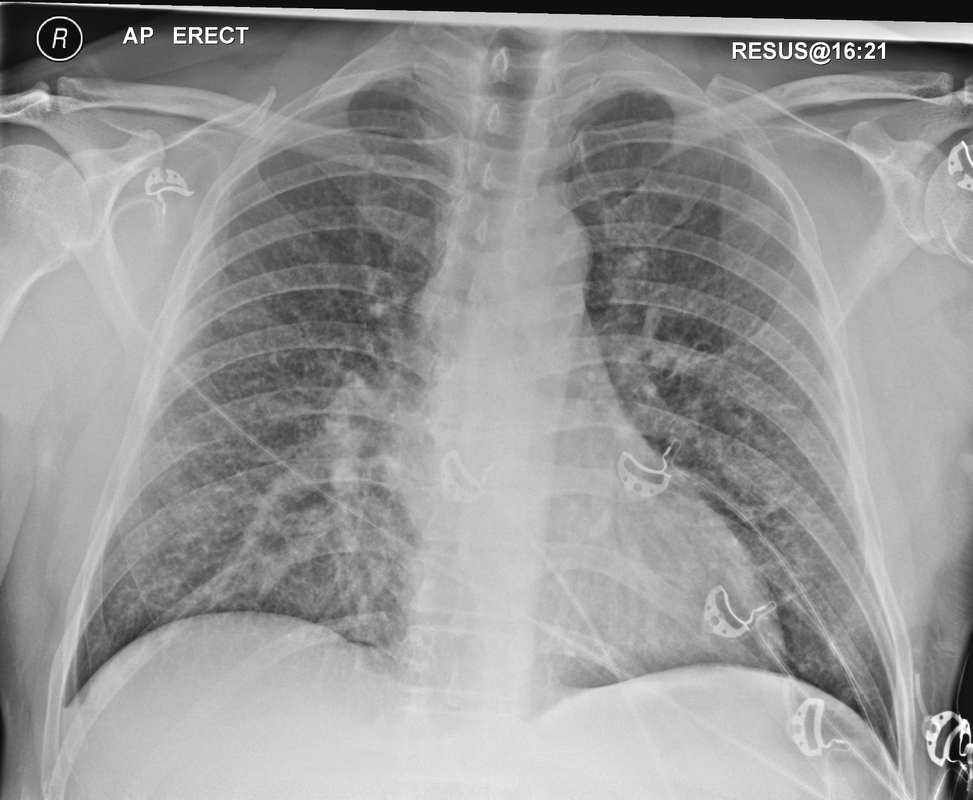
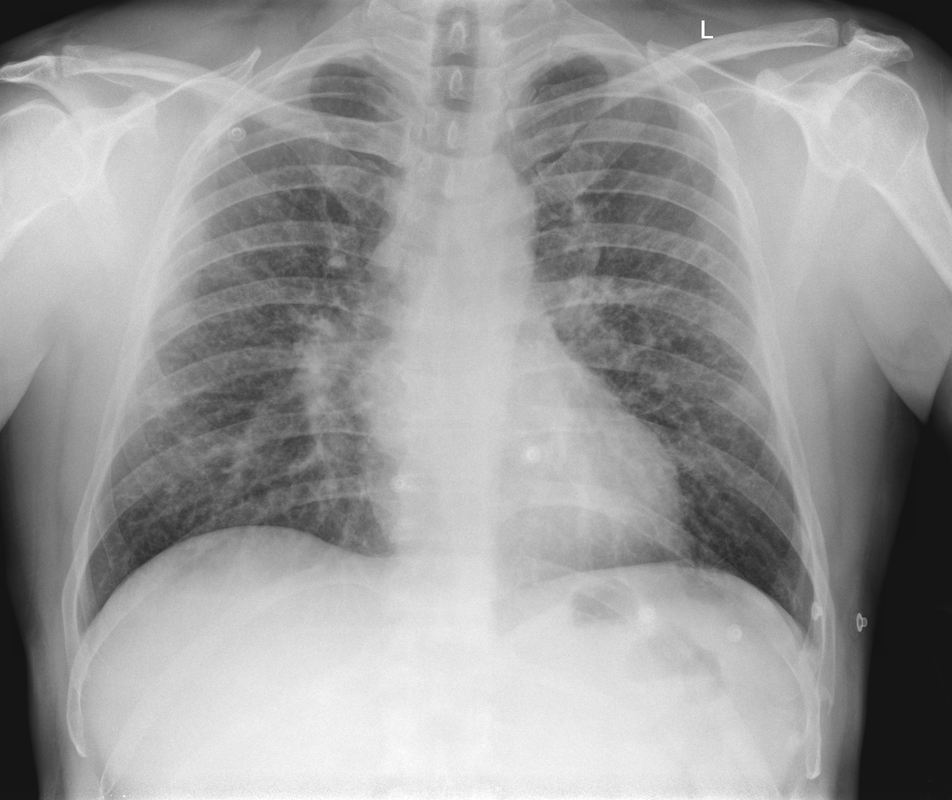
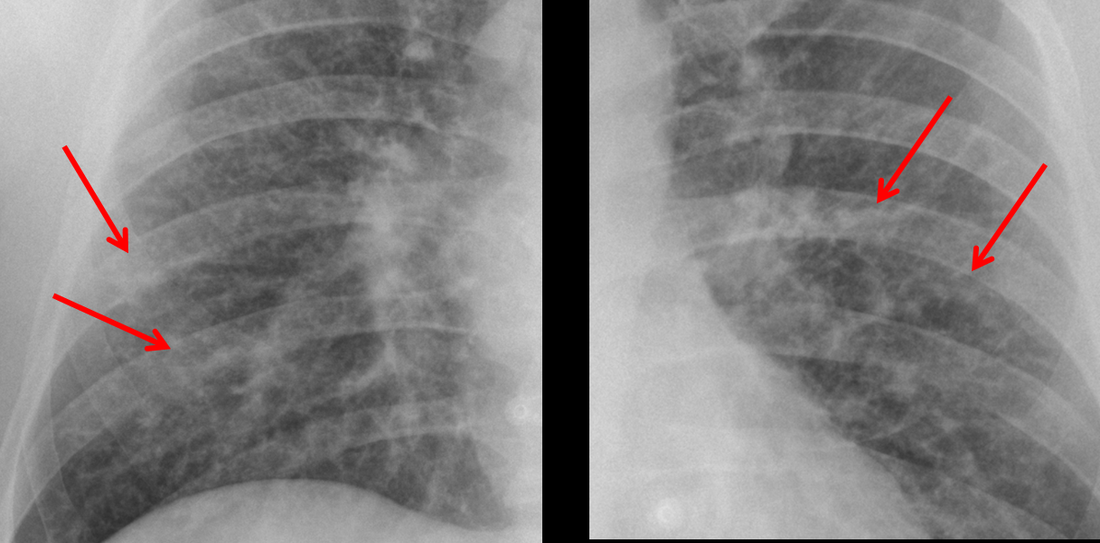
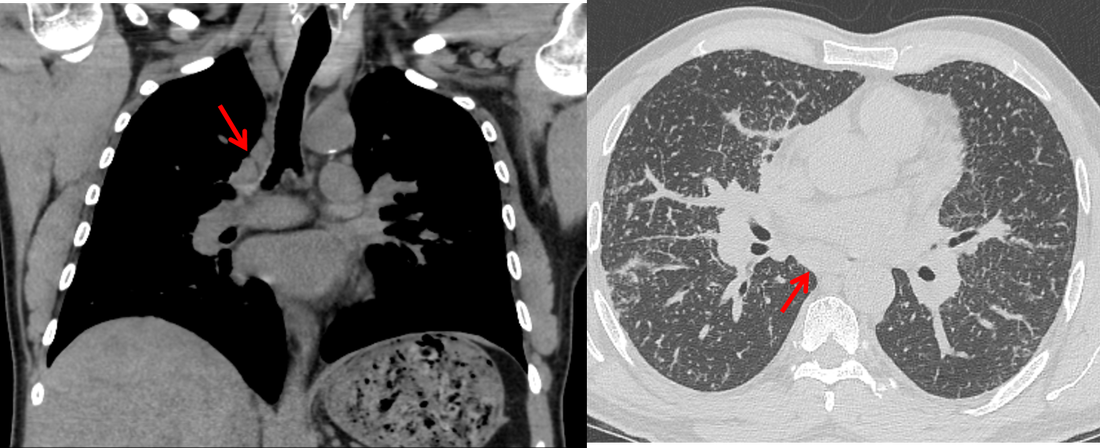
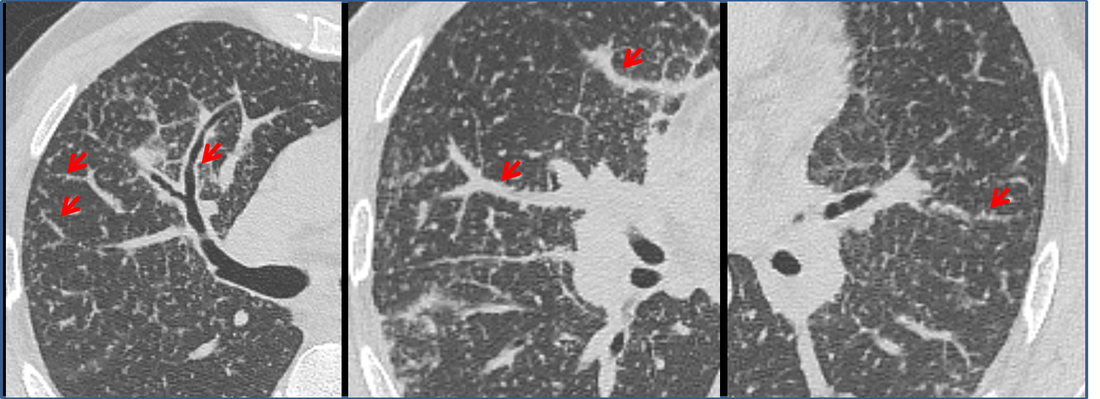
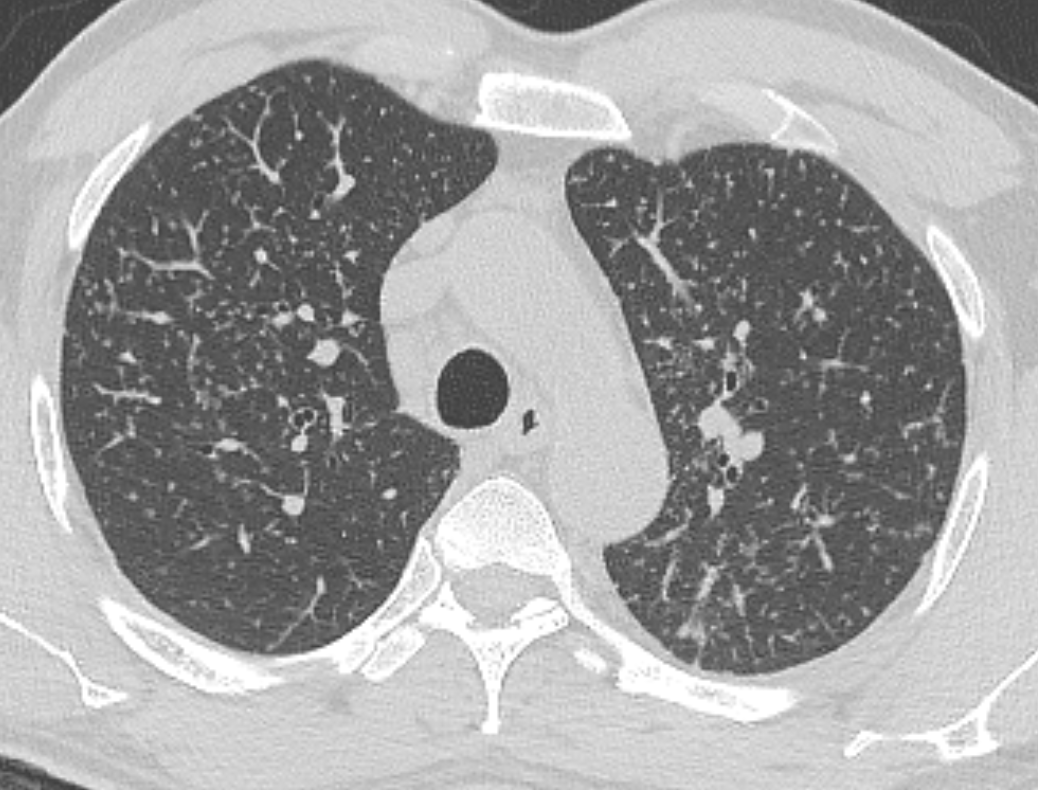
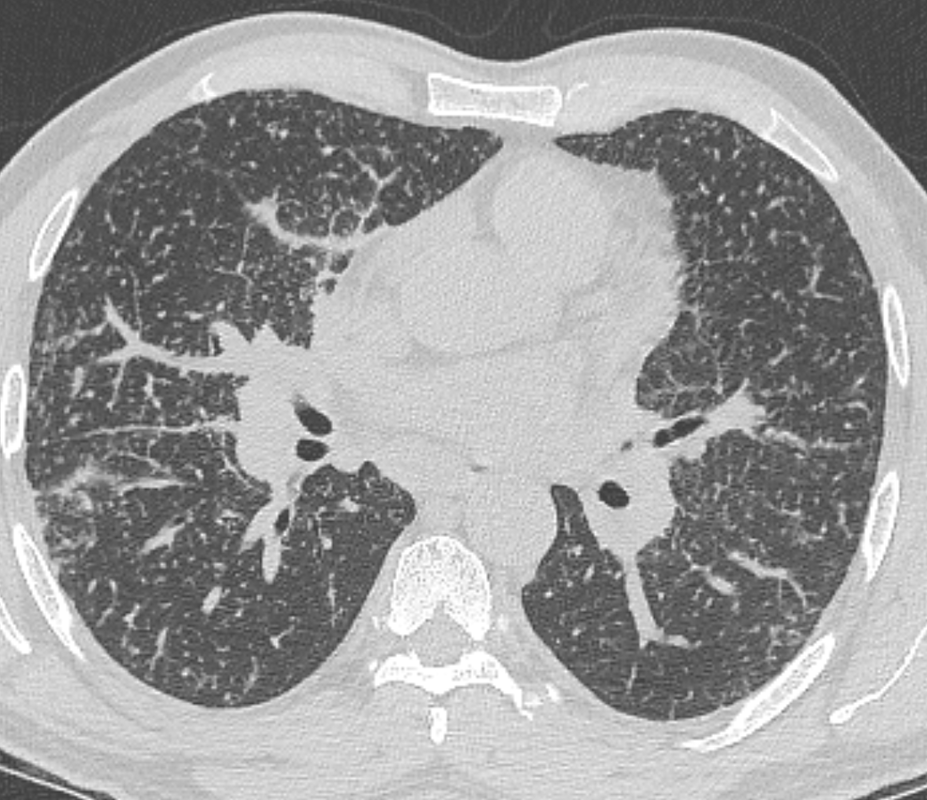
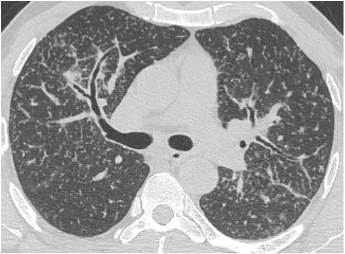
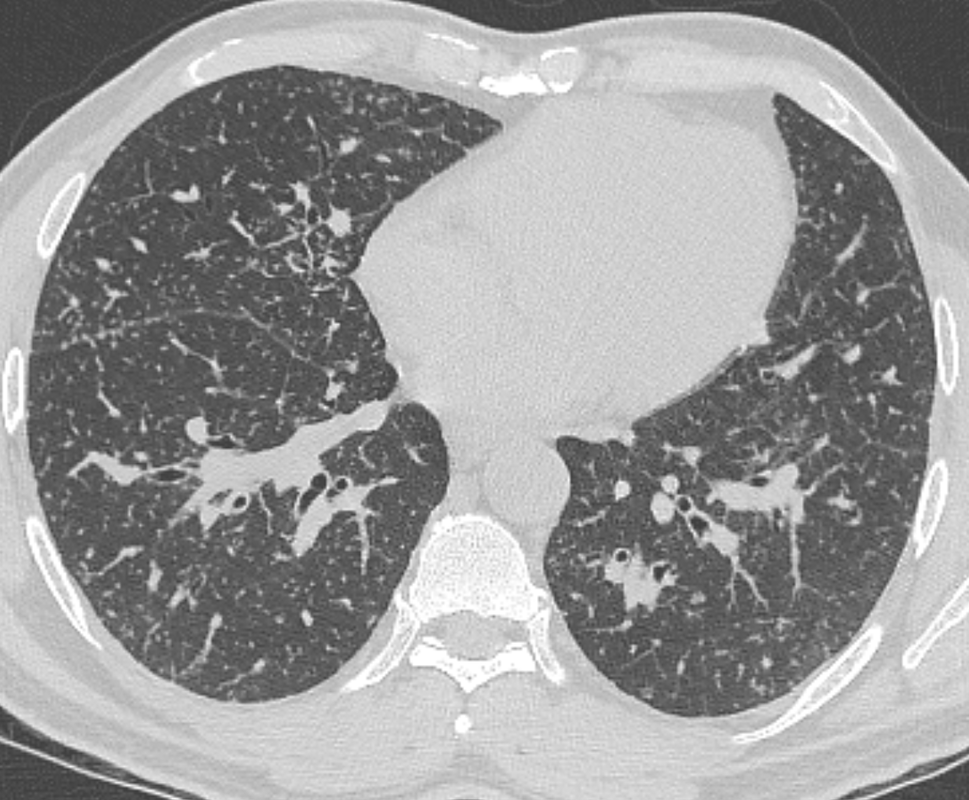
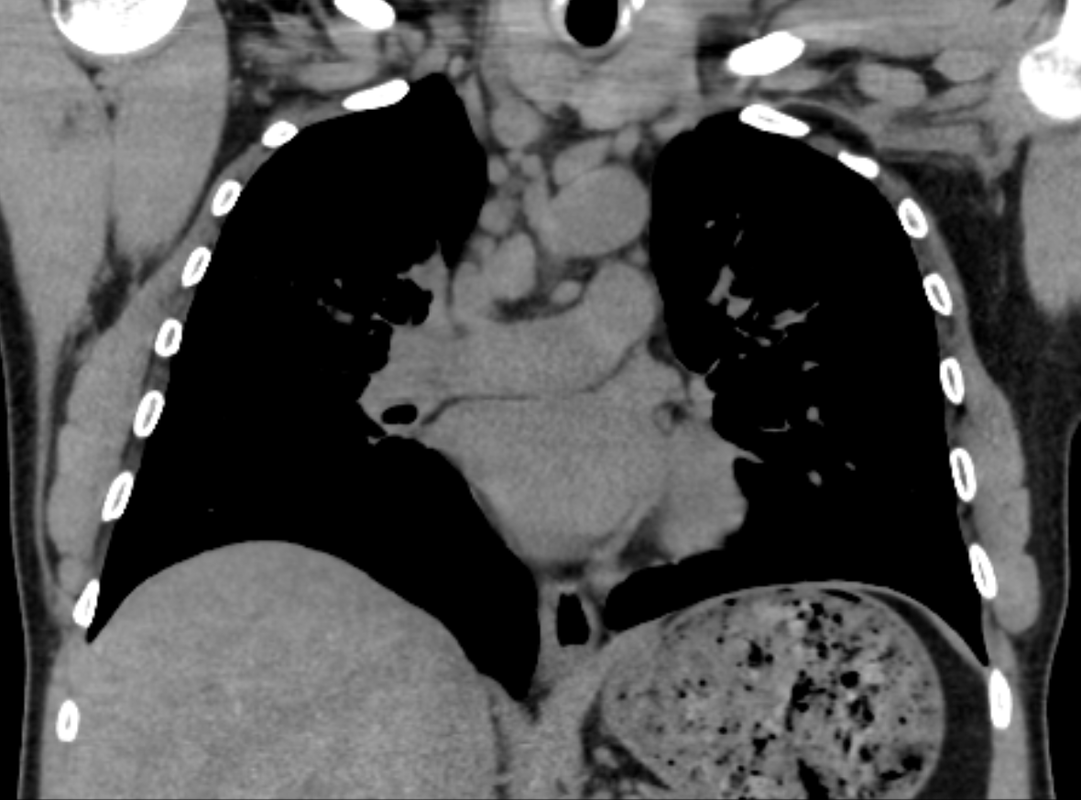
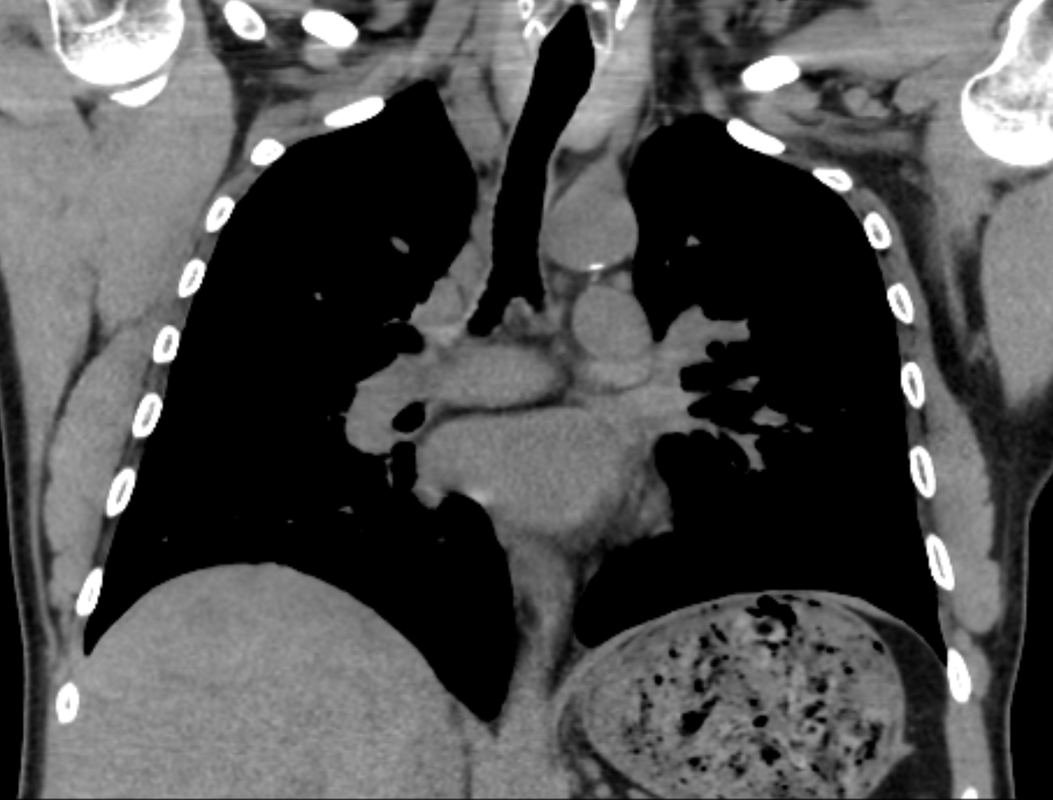
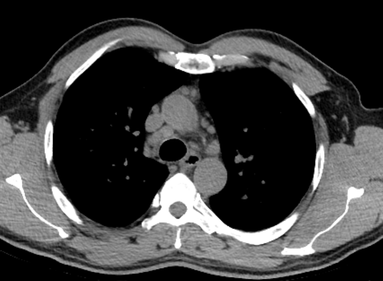
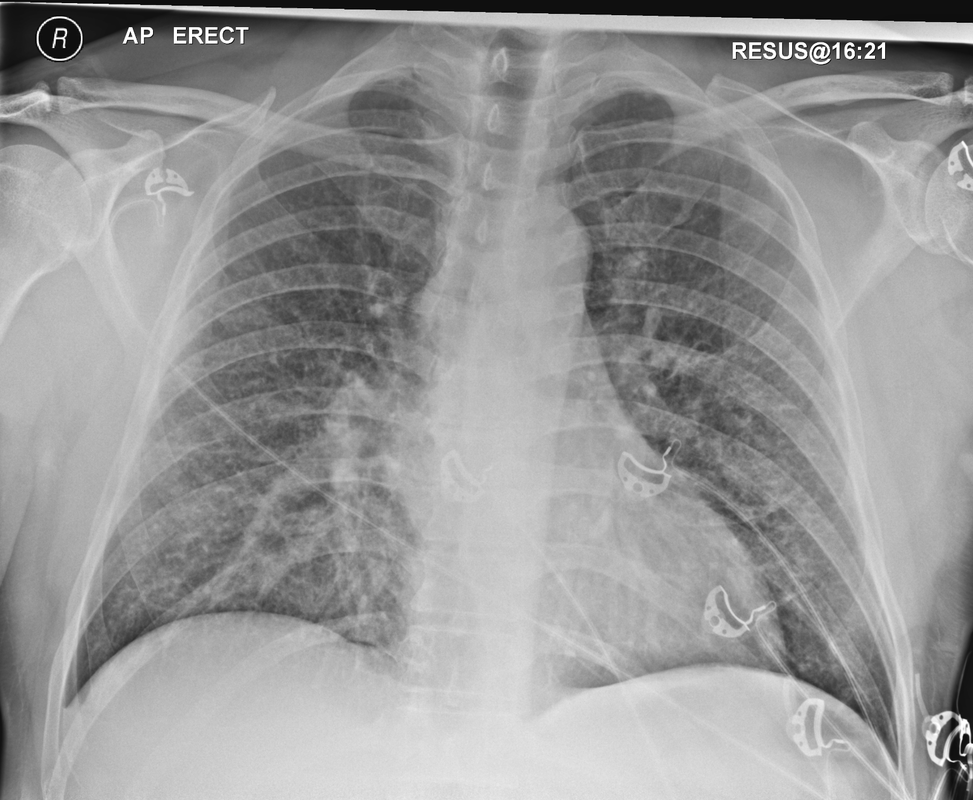
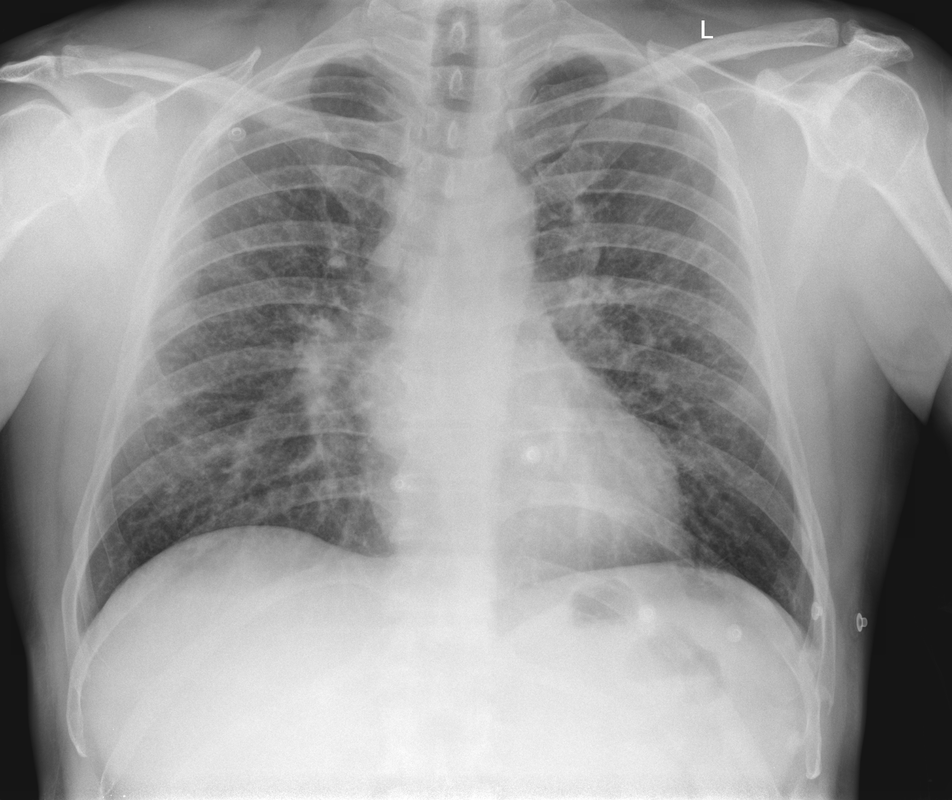
Mar 4th-5th (ONLY 2 PLACES LEFT), Mar 20th-21st (FULLY BOOKED) Mar 11th-12th, Mar 15th-16th Limited availability Written 1 Day Viva Course: Mar 17th Limited availability Radiology Level: FRCR, ABR, EDiR, MRCP, Radiology Mid-Level++ Initial Radiograph The initial AP erect radiograph is covered in ECG leads. This indicates that the patient is unwell, an important factor when considering the causes of abnormality on a chest radiograph. The film demonstrates prominence of the central vasculature, with indistinctness of the central bronchovascular structures and linear markings extending from the hilum. The features suggest interstitial oedema. The upper lobe vasculature is not particularly prominent and the heart is not markedly enlarged, however, these are features of chronic pulmonary venous hypertension. This patient is young and so unlikely to as yet to have developed these features. At aged 35 atrial fibrillation is unusual and the likely cause of interstitial oedema. 2 week follow-up The follow up radiograph is following two weeks of therapy. The ECG leads are gone and the patient was better, clinically out of interstitial oedema. It is tempting to think that the chest radiograph is now normal, or at best suggests some reticular linear opacities radiating from the hilum that may suggest some residual interstitial oedema sequelae. But look more closely at the film, particularly in the mid zones. There are additional nodules present. Indeed these are superimposed over the linear reticular markings so this appearance is what we would call reticulonodular. Look at the lateral margin of the horizontal fissure and some of the larger and smaller vessels, they appear beaded due to adjacent nodules (long arrows). Determining whether an interstitial process is nodular (e.g. miliary), reticular (e.g. fibrotic or oedema) or reticulonodular is critical to determining the cause of pathology and does take some experience. If you saw the nodules but thought this might be miliary nodules in an Asian patient (i.e. perhaps TB), not bad, but miliary is a haematogenous process and should be worst at the bases. For trainees, especially in exams, this is stressful and it is a common situation for trainees to plump for reticulonodular as a compromise where they feel they are not going to be too wrong either way. This is a bad approach. Determining whether the disease is one of these three options helps you to determine the cause. Don’t chicken out of making that decision! In this case reticulonodular meant to me when I was reporting these plain films that I was effectively down to only 2-3 diagnoses, which we will come to. What else on the second chest radiograph? Well the right paratracheal stripe is widened at its inferior margin and the right superior retrocardiac region where the azygo-esophageal stripe should be visible is widened (short arrows). In the tracheobronchial groove the right paratracheal widening could be due to a prominent azygos due to fluid overload/oedema, but the right retrocardiac/azygoesopahageal widening confirmed adenopathy. I was now down to effective one diagnosis. I didn’t have old reference films so I requested a CT with HRCT imaging to confirm my suspicion. Select annotated images from original provided CT set The CT examination was performed a couple of weeks later. It confirms the presence of adenopathy in the right paratracheal region and the azygo-esophageal region (short arrows). Note how on the coronal images the right paratracheal region is widened and causes the chest radiograph appearances. Selected annotated images from the original provided CT set What about the lung parenchyma? There are numerous small nodules present, measuring 2-3mm in size at CT. This is a micronodular appearance. To evaluate this, my first question is whether they contact the fissures or the pleural surfaces. Look at the beading of the major fissures, clearly they do. This means the nodules are lymphohaematogenous and not centrilobular. Now let us examine further. The next question to consider is whether the nodules are diffuse and distributed randomly (i.e. miliary) or whether they are more clustered around the central vessels and airways (the so called axial interstitium) and the fissures/pleural edges. In this case it might be tempted to think that the nodules are everywhere, they must be random. But look carefully at how thickened the central airways are. Look at the fuzzy nodular appearance of the central airways and vessels extending to the periphery. These are studded with innumerable small nodules. Even where nodules appear “random” in the middle of the lung, they are actually lining vessels or airways. This distribution of nodules along vessels, airways, pleura and the fissures is termed a perilymphatic distribution and corresponds to rerticulonodular at plain radiograph interpretation. I am now only considering three diagnoses: sarcoidosis (and its mimic silicosis in appropriate occupational exposure) and lymphangitis carcinomatosa.
How do I narrow it down? Well lymphangitis is more often unilateral or asymmetric. Interlobular septal thickening is far more common, effusions are very common. Neither are present in this case. So I am left with sarcoidosis or silicosis. I could go and find out if the patient had an occupational exposure, but I am a radiologist and need to push the diagnosis from imaging and clinical knowledge. Firstly silicosis is increasingly rare unless I am in a mining/sandblasting community. Nodes are more likely to calcify in silicosis, although could be non-calcified in both entities. But more importantly this person presented with atrial fibrillation with interstitial oedema at a young age. Silicosis does not cause cardiac disease. Sarcoidosis itself does not usually result in effusions. However, when in sarcoidosis you see evidence of arrhythmia, effusions or oedema, always think about the subset of sarcoid patients who have additional cardiac sarcoid. The diagnosis in this case was indeed sarcoidosis with pleuroparenchymal and cardiac involvement. I made the diagnosis based on the two chest x-rays but it was academically gratifying to follow through to see the CT imaging and make an illustrative teaching case. Maybe at another time we will look at the next investigation that should follow, a cardiac MRI to confirm myocardial sarcoidosis. Radiology Level: FRCR, ABR, EDiR, MRCP, Radiology Junior +
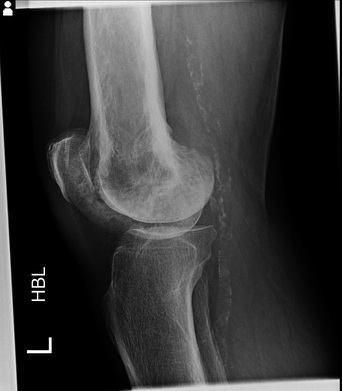
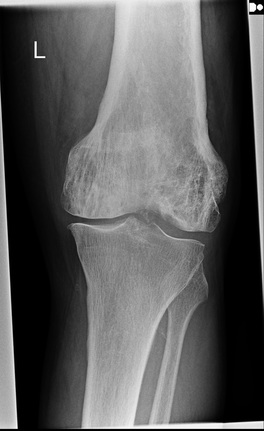
This is a relatively simple case. The lateral and AP projection of the knee demonstrate minor degenerative changes of the knee including the patellofemoral joint. However, there is an abnormality of the distal femur. The femur demonstrates patchy diffuse sclerosis. This extends extensively beyond the articular margin of the joint and is, therefore, not related to a degenerative process. The patchy appearances may suggest an infiltrative disorder such a metastasis or a primary osteogenic lesion. However, there are other features here that suggest an alternative diagnosis. The distal femur demonstrates thickening and coarsening of the medullary trabeculae. The cortex of the distal femur is also considerably thickened compared to the cortex of the femur. Further one can appreciate that the femur size is subtly enlarged compared to the tibia. Finally, the entirety of the femur is involved extending to involve all of the femoral condyles to the articular surface. These are classical appearances of Paget’s disease. Paget’s disease typically involves the entirety of a single bone extending from one articular surface to the other side of the bone. The typical appearances seen here those of coarsening of the trabeculae, cortical thickening, sclerosis and osseous enlargement. Bowing may then develop in the long bones. This is typically lateral in the femur but anterior in the tibia. Premature degenerative change is also a feature in long bones. Paget’s disease most typically involves the pelvis although involvement of other sites including the skull, proximal long bones and vertebrae is also common. Both poly-ostotic and mono-ostotic variations can occur. The mixed osteosclerotic and osteolytic phase observed here is preceeded in some patients by a pure osteolytic phase that is often not clinically detected. This may in the long bones be associated with an advancing margin often likened to a “blade of grass” or the “candle flame sign”. A later fully sclerotic osteoblastic phase may occur. Although there is often extensive sclerosis the serum calcium and phosphate levels are normal with increase in serum Alkaline Phosphatase or urinary hydroxyproline the only consistent biochemical findings. Clinically approximately 75% of patients are incidentally detected and asymptomatic, although local pain and even symptoms of increased heat sensation can occur with long-bone involvement. Long term the principle concerns of long bone involvement are deformity, fractures, and in approximately 1% of patients, osteosarcoma transformation. Treatment is with bisphosphonate therapy. 2 day courses:
Mar 20-21 course now fully booked Mar 4-5 course only 3 places left Limited availability for Mar 11-12 and Mar 15-16 1 day written course: Limited availability for Mar 17 |
From Grayscale
Latest news about Grayscale Courses, Cases to Ponder and other info Categories
All
Archives
October 2018
|

|
|
Grayscale Courses est. 2015
 RSS Feed
RSS Feed
