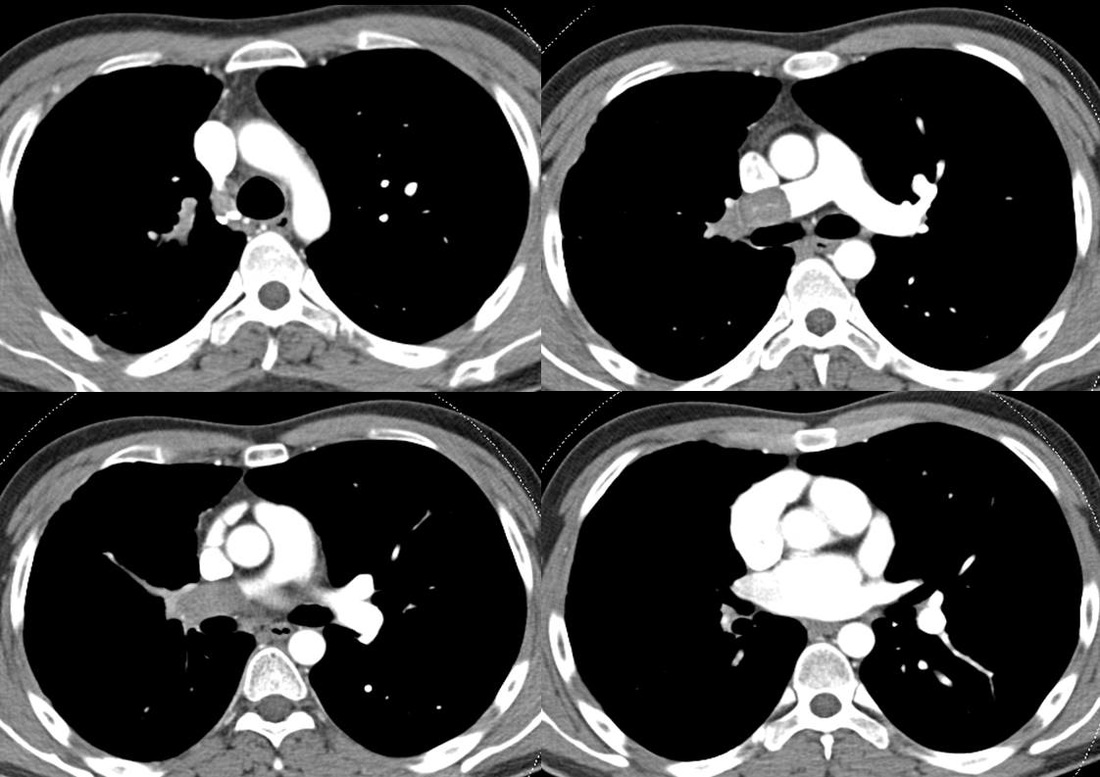
The first four images are interpreted as per normal CT. These demonstrate a large filling defect in the right main pulmonary artery extending into the peripheral arteries of the right upper and lower lobes. So this is a pulmonary embolism, right? Well let’s look carefully at the images, anything else? Well the peripheral pulmonary arteries are rather small on the right and large on the left. There are also bronchial artery collaterals present around the trachea and carina in the first two images. These are features of chronicity. Let’s also now look more carefully at the central thrombus in the second image. There is a subtle serpiginous high attenuation tract in the middle of it.
At this point, one might consider that this filling defect reflects a chronic thrombus. Most of these findings would certainly fit although one might expect the central thrombus to be more retracted to the surface, for there to be other webs or bands in the pulmonary arteries and the main pulmonary artery to be larger indicating pulmonary hypertension. Perhaps the serpiginous high attenuation tract reflects recanalisation, although we do not see clear communication of the serpignous high attenuation to the lumen.
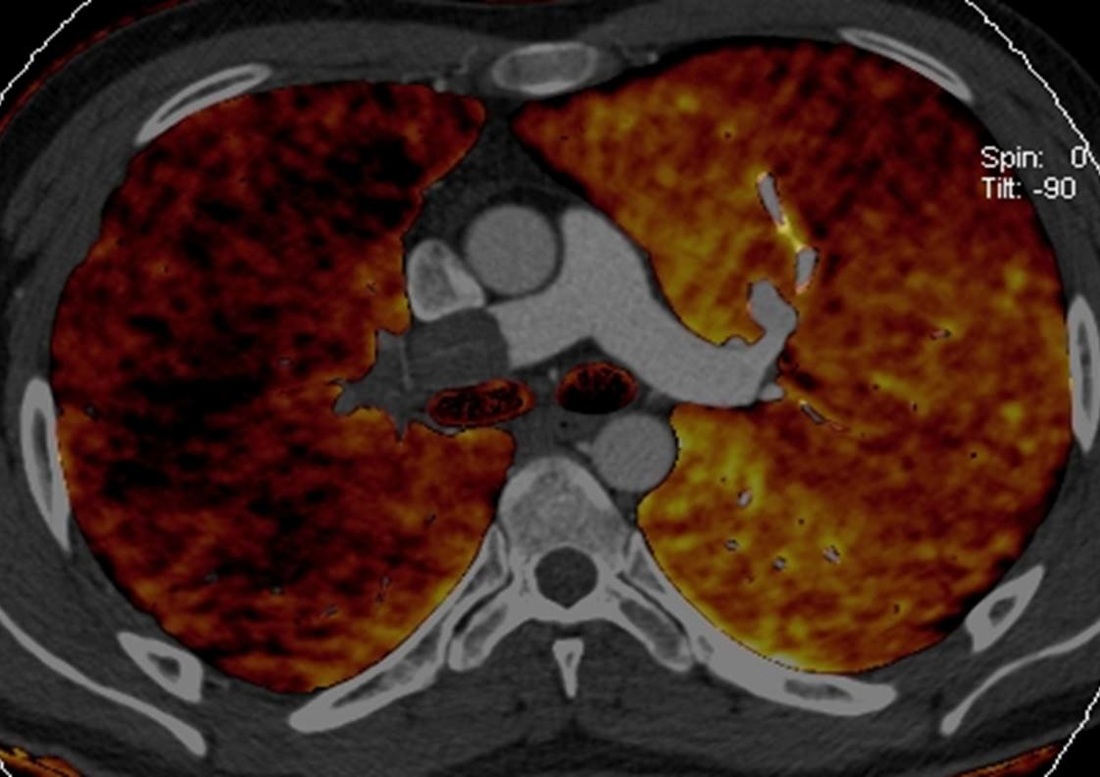
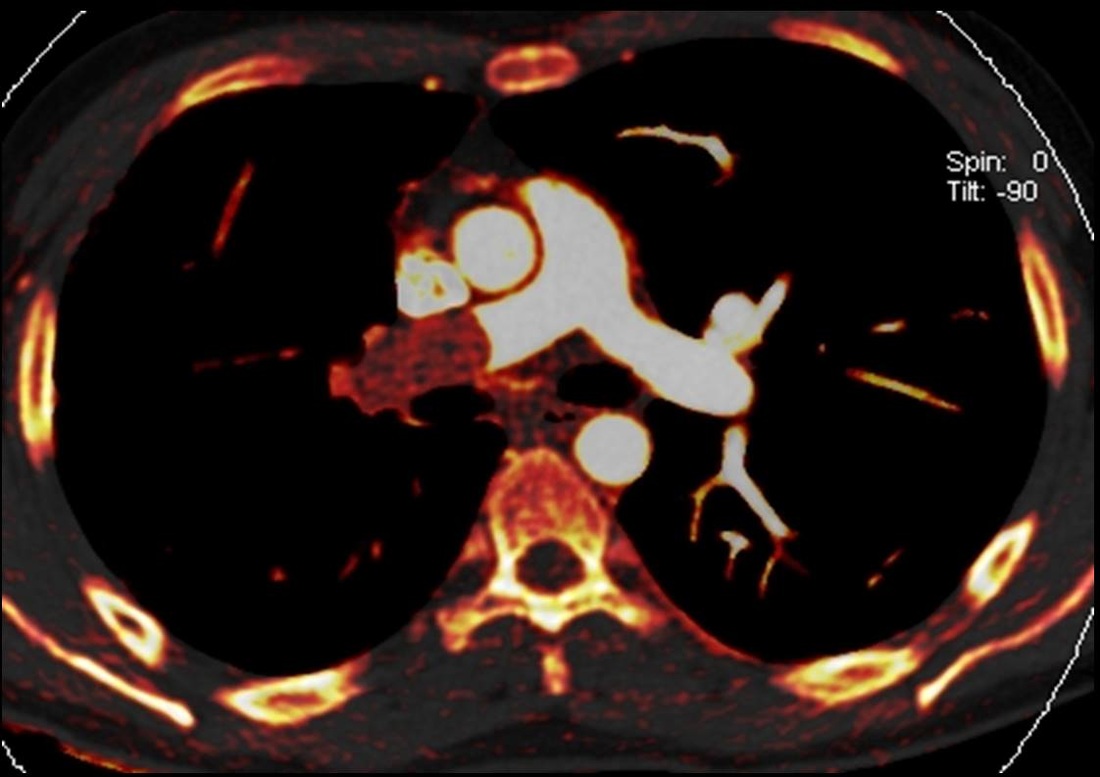
Let us now look at the two dual energy images (or spectral images – these are synonyms). These images are created by using a scanner capable of scanning simultaneously at two different energies. This can be by a dual source dual detector system (Siemens), a single source system with rapid kVp switching (GE), or a thick “sandwich detector” the superficial portion detecting low energy and the deeper portion the high energy photons (Philips). Whichever the technique, the principles are the same. A scan is acquired simultaneously at 80-100kVp and at 140kVp. Simplistically, by scanning materials at different energies, it is possible to determine the material composition of a CT image. In these 2 images the iodine distribution has been colour coded in the lung parenchyma (pulmonary blood volume) in the first and in the soft tissues in the second.
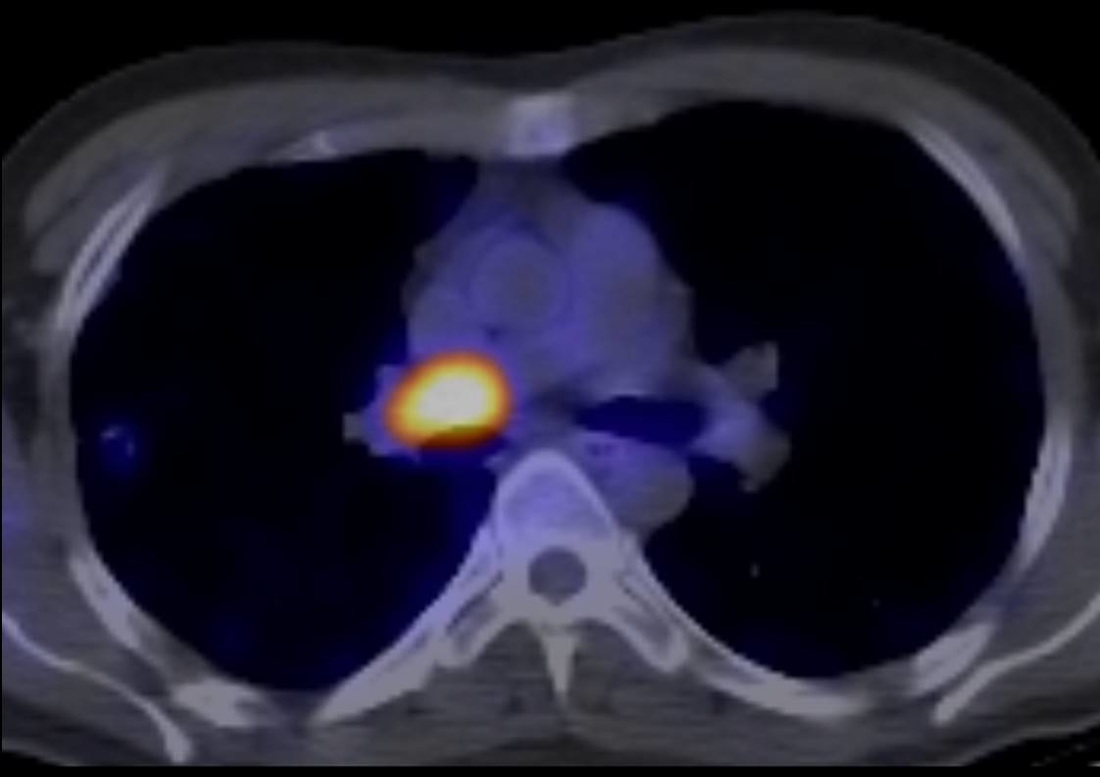
The first dual energy image shows diffuse reduction in perfusion of the right lung. This is not the variable mosaic pattern one would expect for chronic thromboembolic disease. The second image is more difficult to evaluate for novices to this technique. This demonstrates intermediate enhancement (orange) in the pulmonary artery filling defect. This is indicative of enhancement within the pulmonary artery filling defect – a bland PE would be black. The serpiginous high attenuation tract can now be realized to be a vessel supplying an enhancing tumour. Although metastatic large lesions can deposit in the pulmonary arteries, primary lesions are commoner, typically leiomyosarcoma or other sarcoma variants. Indeed in this case this is a leiomyosarcoma, the biological activity of which is confirmed by the final PET-CT image.
Leiomyosarcoma of the pulmonary arteries can be a difficult diagnosis to make if the tumour does not invade outside the pulmonary artery walls, which unfortunately is the commoner pattern. Clinically these patients may present with acute symptoms of PE or a more indolent course. There may be a history of large PE unresponsive or only partially responsive to anticoagulation or thrombolytics, the latter due to resolution of adherent bland clot. Radiologically clues include the presence of a large filling defect, with heterogeneity of attenuation or cystic changes. If precontrast or dual energy images are available these can be used to show enhancement which does not occur in normal bland thrombus. The diagnosis is often missed initially and should be considered on repeat examinations when there is lack of clinical progress. In this case the patient underwent pneumonectomy and had a good clinical course.

 RSS Feed
RSS Feed
